BACKGROUND: Men being treated for benign prostatic hyperplasia (BPH) may have reduced quality of life (QoL) due to persistent overactive bladder (OAB) symptoms. We report health-related QoL (HRQL) outcomes from a phase 3 trial of vibegron, a selective β3- agonist under investigation for use in men with pharmacologically treated BPH and persistent OAB symptoms.
METHODS: COURAGE (NCT03902080) was a 24-week, phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Men ≥45 years with OAB and BPH receiving a stable dose of α-blocker ± 5α-reductase inhibitors were randomized 1:1 to once-daily vibegron 75 mg or placebo. QoL was assessed by the overactive bladder questionnaire (OAB-q) long form, comprising the symptom bother subscale score and the HRQL total score (including coping, concern, sleep, and social interaction subscores), with 1-week recall. Change from baseline (CFB) at Week 12 and 24 was analyzed using a mixed model for repeated measures.
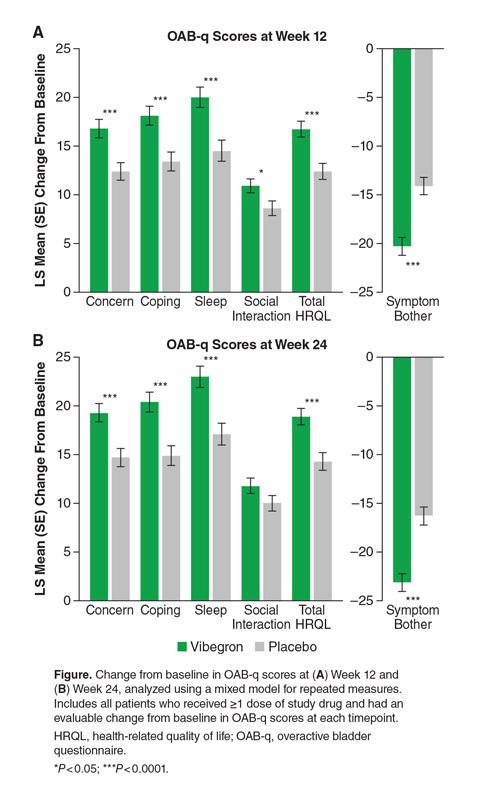
RESULTS: Overall, 969 patients (vibegron, n=487; placebo, n=482) and 923 patients (vibegron, n=464; placebo, n=459) had evaluable CFB in OAB-q scores at Weeks 12 and 24, respectively. Baseline OAB-q scores were similar between treatment groups. Vibegron was associated with significant improvements from baseline at Week 12 vs placebo in symptom bother score (LS mean [SE] CFB, −20.3 [0.87] vs −14.1 [0.87]; LS mean difference [LSMD], −6.2; P<0.0001) and total HRQL score (16.7 [0.83] vs 12.4 [0.83]; LSMD, 4.2; P<0.0001), as well as in the concern (16.8 [0.91] vs 12.4 [0.91]; LSMD, 4.4; P=0.0001), coping (18.1 [0.97] vs 13.4 [0.97]; LSMD, 4.7; P=0.0001), sleep (20.0 [1.06] vs 14.5 [1.07]; LSMD, 5.5; P<0.0001), and social interaction (10.9 [0.75] vs 8.6 [0.75]; LSMD, 2.2; P=0.0175) subscores (Figure). Improvements remained significant at Week 24 (P<0.0001) for all scores except social interaction.
CONCLUSIONS: Vibegron was associated with improvements vs placebo in OAB-q scores at 12 and 24 weeks, indicating that vibegron treatment improved quality of life among men with pharmacologically treated BPH and residual OAB symptoms, consistent with the improvements in micturition frequency and urgency observed in this phase 3 trial.