BACKGROUND: Intravesical therapy is the gold standard for intermediate- or high-risk non-muscle invasive bladder cancer (NMIBC). The most-studied therapy is Bacillus Calmette-Guerin (BCG), however, despite more than 40 years of clinical use, the patient-reported toxicity of treatment has not been defined. This is relevant, as roughly 20% of patients will not complete the induction BCG course due to toxicity. There is also an ongoing shortage which has prompted investigation of other intravesical therapy regimens. These are also used in the BCG-unresponsive setting. To date, no patient-reported assessment of toxicity during therapy has been described for BCG or alternative intravesical therapies. Our objective was to use a validated measure to compare longitudinal toxicity throughout intravesical therapy.
METHODS: Patients treated a Memorial Sloan Kettering between June 2022 to September 2023 were automatically sent a questionnaire through the electronic patient portal at the time when intravesical therapy was ordered, and at three days following each scheduled treatment (with a 2-day window to complete). Data collection is ongoing, with a planned re-analysis in May 2024. This was IRB approved for research use, with no change in clinical care planned based on the responses. To measure the toxicity of therapy, we chose 18 relevant items from the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). We grouped these adverse events into constitutional, cardiopulmonary, gastrointestinal, mental, and urinary categories. Toxicity grades range from 0-3. Natural cubic splines were fit to mean scores to visualize outcomes. Linear mixed effects models, adjusting for clinical factors, were used to compare longitudinal toxicity.
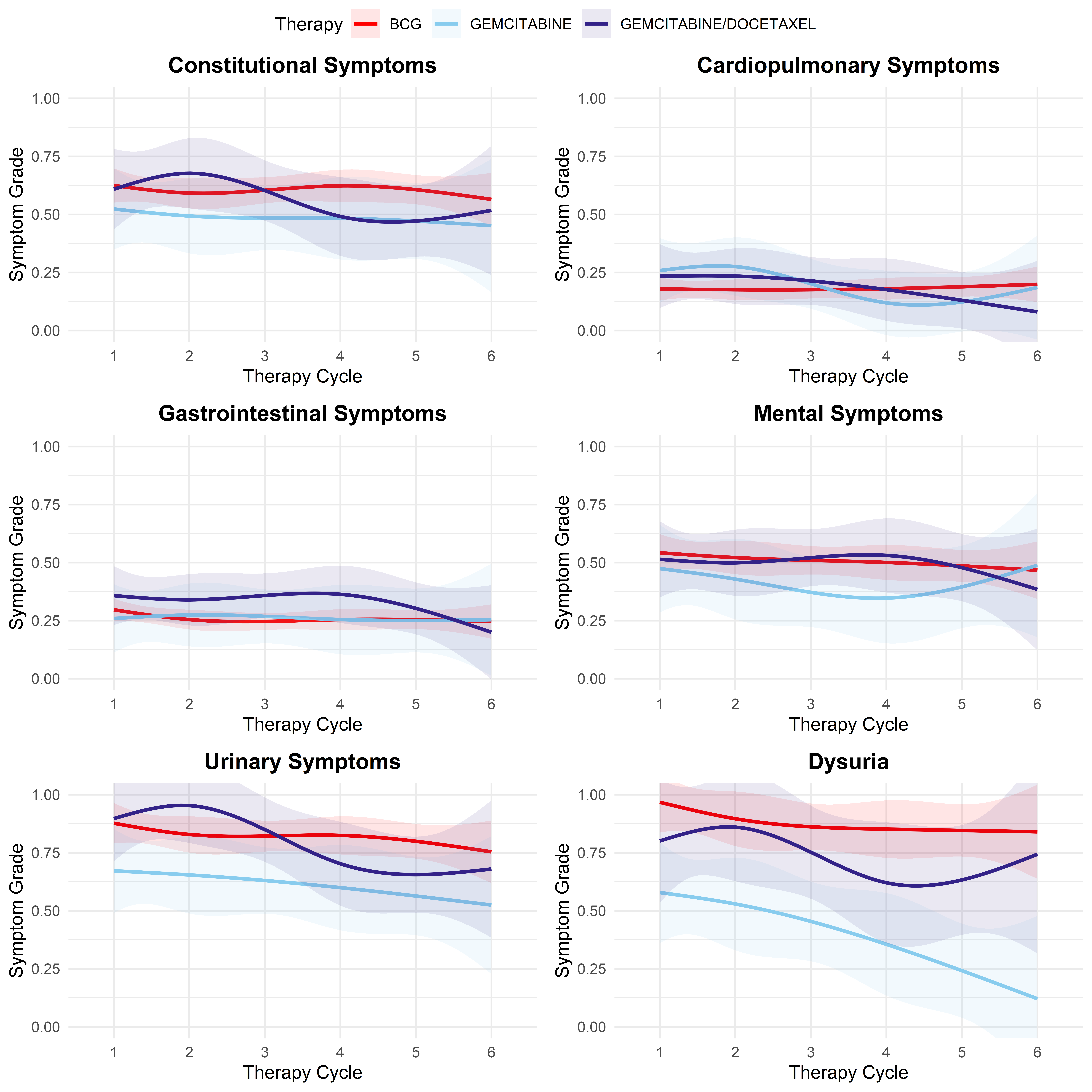
RESULTS: A total of 267 subjects completed at least one survey during therapy (n=199 BCG, n=35 gemcitabine, n=29 gemcitabine/docetaxel). Only 4 received mitomycin and were not analyzed due to low number. A median of 5 surveys were completed per subject. There was no evidence of a significant difference in constitutional symptoms between BCG and gemcitabine (p=0.16) alone or gemcitabine/docetaxel (p=0.54). Urinary symptoms overall were similar (gemcitabine p=0.09, gemcitabine/docetaxel p=0.5), but for Dysuria, toxicity was greater for BCG - with a coefficient of -0.65 (p<0.001) for gemcitabine and -0.32 (p=0.048) for gemcitabine/docetaxel. There was no evidence of significant differences in gastrointestinal (gemcitabine p=0.56, gemcitabine/docetaxel p=0.51) mental (gemcitabine p=0.38, gemcitabine/docetaxel p=0.78), or cardiopulmonary (gemcitabine p=0.70, gemcitabine/docetaxel p=0.42) symptoms. Overall, toxicity was low, with mean toxicity grades <1 (maximum 3) for all categories over 6 weeks (Figure).
CONCLUSIONS: Toxicity was low during therapy for BCG, gemcitabine, and gemcitabine/docetaxel - the three most commonly used therapies. Contrary to expectation, there wasn’t evidence of progressive toxicity later in the induction therapy course. Dysuria was the only symptom that was meaningfully greater with BCG compared to the other therapies.