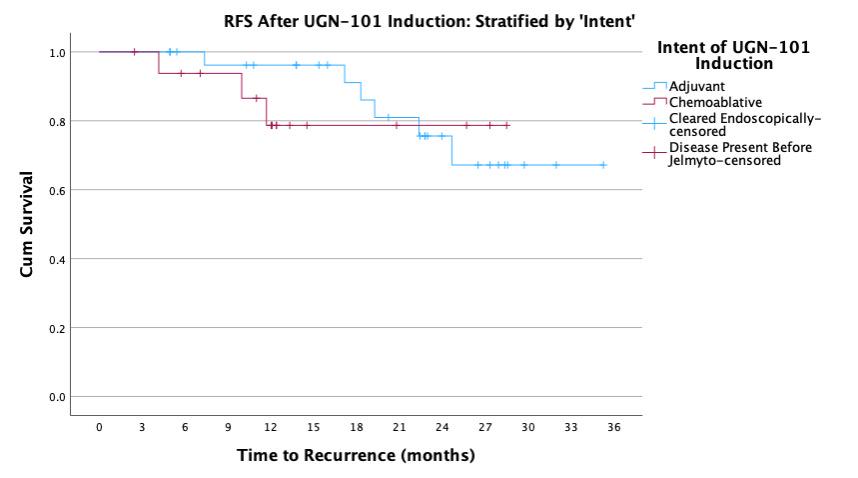
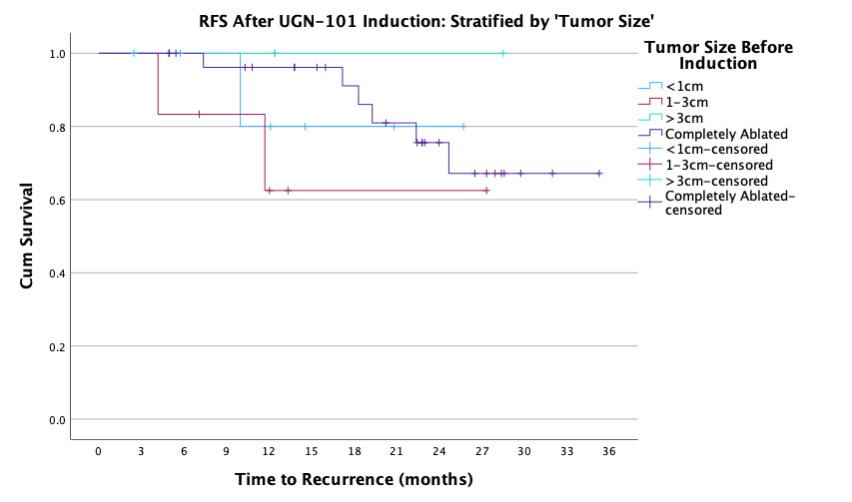
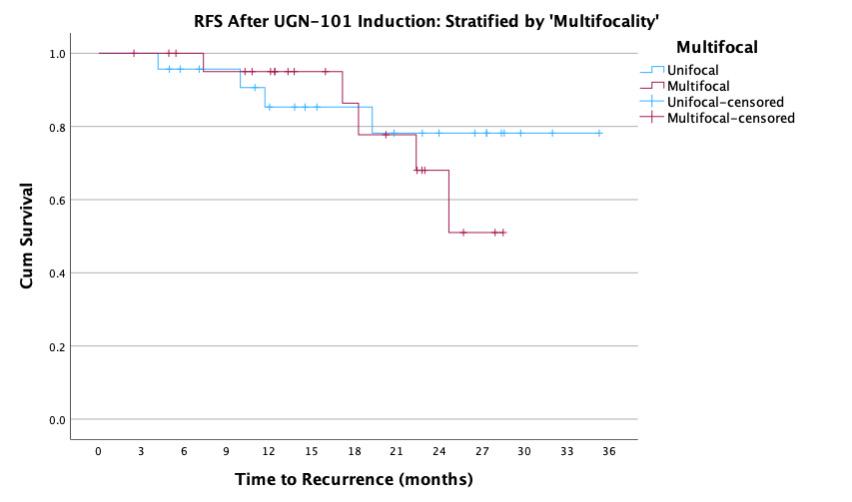
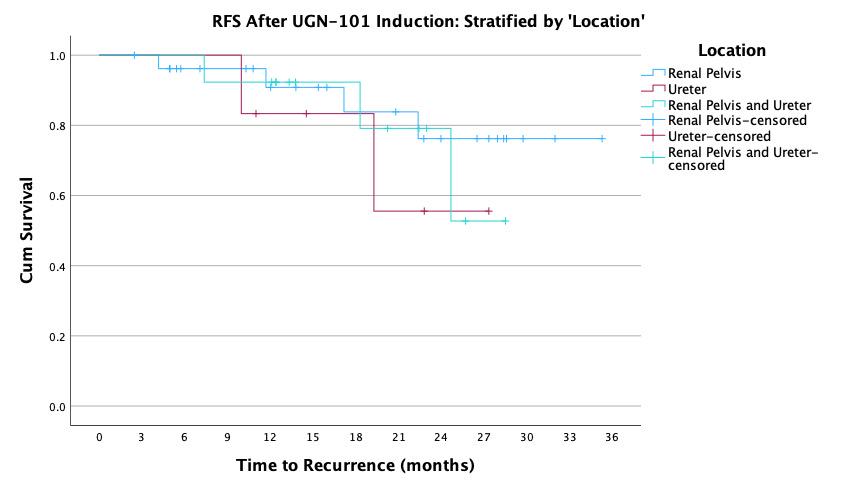
BACKGROUND: UGN-101 is a novel therapeutic for the treatment of upper tract urothelial cancer (UTUC) that was originally approved for chemoablative use in low-volume, low-grade disease of the pelvicalyceal system. In practice, UGN-101 is being utilized more broadly - including for bulky tumors, in the adjuvant setting, and in cases of ureteral involvement. This study aims to evaluate recurrence in these settings. METHODS: Data was collected from 15 centers on patients treated with UGN-101 for UTUC. Recurrence free survival (RFS) was calculated only patients who had no evidence of disease following UGN-101 induction. Chemoablative use was defined as receipt of UGN-101 induction in the setting of known residual UTUC, while adjuvant use was defined as receipt of UGN-101 following visually complete endoscopic ablation. Exploratory analyses were performed to assess impact of size of tumor, presence of ureteral involvement, and multifocality of UTUC prior UGN-101 induction. RESULTS: There were 136 cases of UTUC treated with UGN-101 with a cumulative median (IQR) follow up of 22 (12-27) months including 107 cases of LGTa UTUC. After initial treatment, 74% of adjuvant and 39% of chemoablative patients were disease free totaling 53 cases with LGTa UTUC without evidence of disease following UGN-101 induction. Median recurrence-free survival was not reached and RFS at 24-months was 86%. Among these patients, there was no difference in RFS based on initial disease characteristics (size, location, multifocality) and treatments according to chemoablative vs. adjuvant intent of UGN-101 induction (log rank 0.597). CONCLUSIONS: UGN-101 treatment appears to demonstrate favorable recurrence free survival rates for patients with LGTa UTUC who respond to initial induction. There does not appear to be a recurrence difference according to the intent of UGN-101 induction, original tumor size, multifocality or tumor location.