BACKGROUND: A single randomized trial (i.e., the POUT trial) has examined the role of adjuvant chemotherapy (AC) for locally advanced (pT2-4 or N+) upper tract urothelial carcinoma (UTUC) after radical nephroureterectomy (RNU), reporting improved survival with AC. However, this survival benefit was not observed in pT2 or N+ patients, albeit with limited subgroups. Based on this limitation and with the lack of real-world data, we therefore emulated the completed POUT trial using a large, nationwide cancer registry.
METHODS: Based on POUT trial eligibility criteria, we identified patients aged 50-79 years with pT2-4 or pN+ non-metastatic UTUC treated with RNU from 2006 to 2015 in the NCDB. A propensity score for receipt of multiagent AC within 3 months of RNU was estimated using logistic regression, and the associations of AC with overall survival (OS) were evaluated after reweighting by stabilized inverse probability of treatment weights (sIPW), with 3-month landmark time analyses. Heterogeneity of treatment effects was evaluated by adding treatment-covariate interaction terms for pre-specified characteristics.
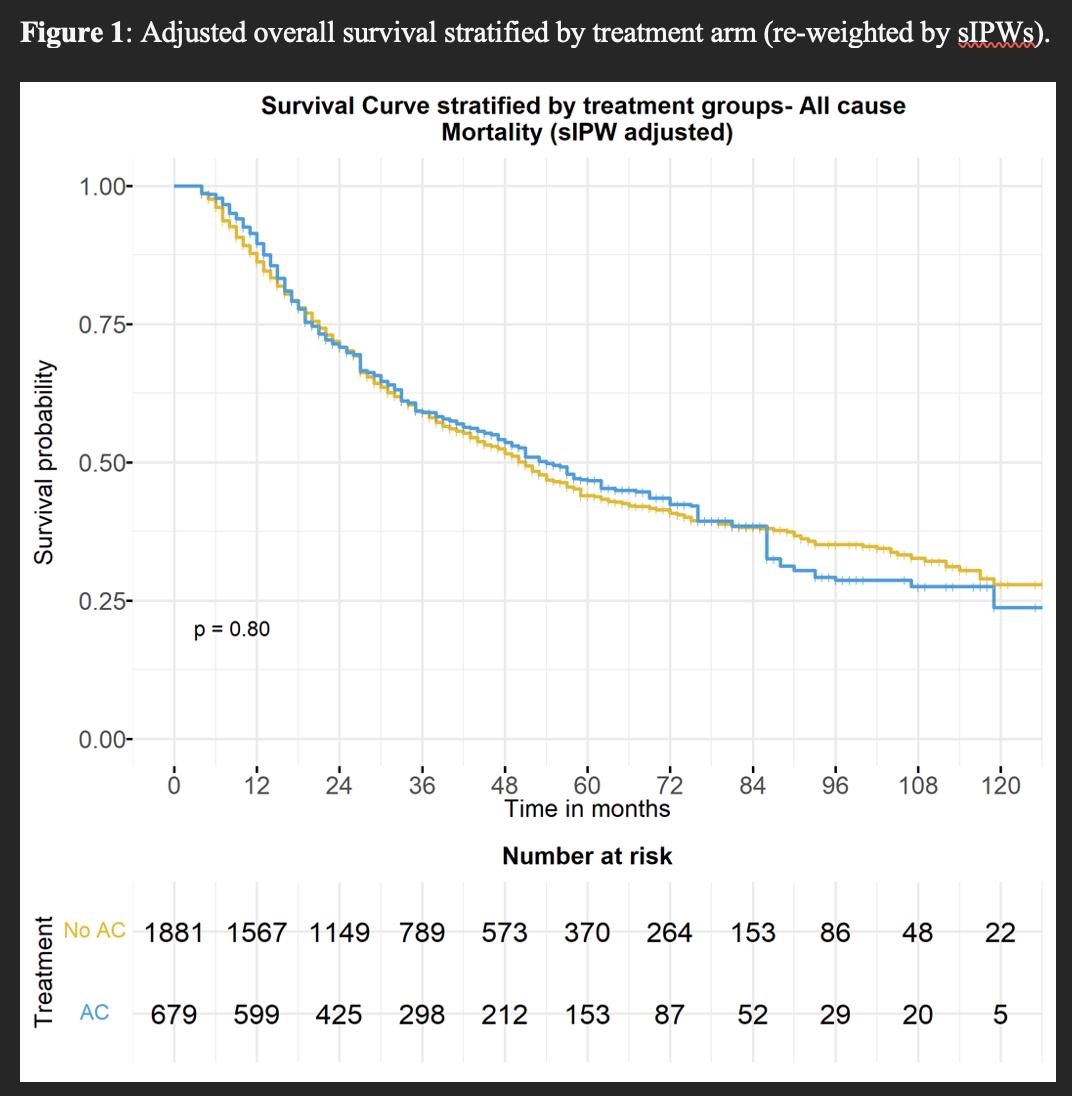
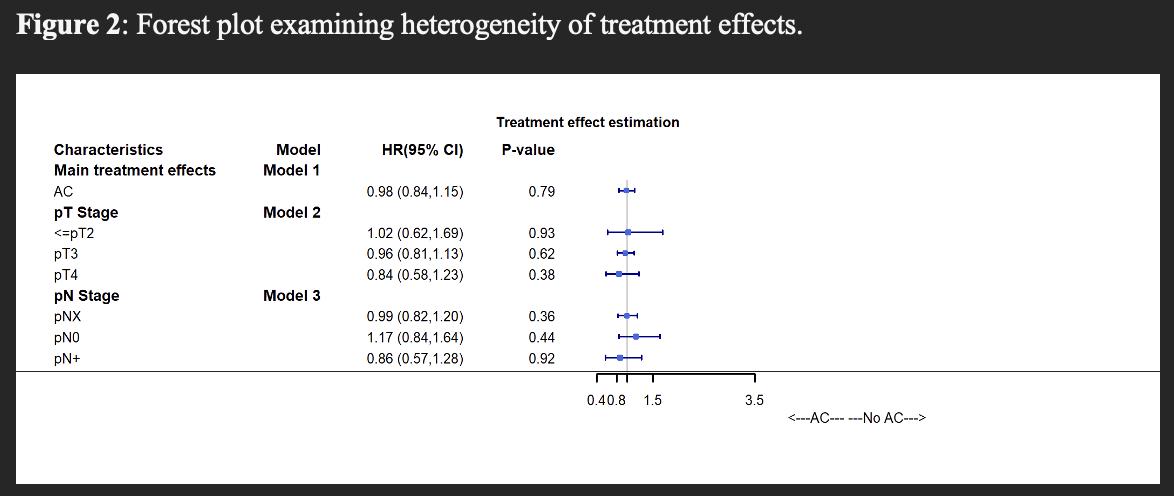
RESULTS: The study cohort comprised 2,537 patients, of whom 719 (28%) received AC within 3 months after RNU. pT stage was ≤pT2 in 668 (26%) patients, pT3 in 1,661 (65%) patients, and pT4 in 208 (8%) patients. A total of 292 (12%) patients had pN+ disease. After adjustment, baseline characteristics were well balanced. Median follow-up was 30.0 (IQR 16.0-54.0) months. After IPW-reweighting, AC was not associated with improved OS compared to observation, with 5-year OS of 47% vs 44%, respectively (HR 0.98, 95% CI 0.84-1.15, p=0.79; Figure 1). When examining treatment effects across baseline characteristics, AC was not associated with improved survival for any pT or pN category (Figure 2).
CONCLUSIONS: In analyses designed to emulate the POUT trial, we did not observe improved OS with AC in the overall cohort, nor in specific pT or pN categories. Notwithstanding potential limitations related to lack of data regarding specific chemotherapy agents utilized and unmeasured confounding, these findings suggest the need to examine the real-world comparative effectiveness of AC in locally advanced UTUC in further studies