BACKGROUND: The ROBUST III study is a randomized controlled trial comparing the OptilumeŽ Drug Coated Balloon (DCB) against standard of care direct visual internal urethrotomy (DVIU) or dilation. The OptilumeŽ DCB is a dilation balloon with a paclitaxel coating that combines mechanical dilation for immediate symptomatic relief with local drug delivery to maintain urethral patency. Outcomes after 4-year follow-up are presented here.
METHODS: 127 subjects were randomized in a 2:1 fashion at 23 sites. Seventy-nine were treated with the DCB and 48 were treated with DVIU or dilation. Follow-up past 1 year was limited to those treated with the DCB. Eligibility criteria included adult males with anterior strictures with ≥2 prior treatments, urethral lumen ≤12F and stricture length ≤3cm. Long-term endpoints included freedom from repeat treatment, International Prostate Symptom Score (IPSS), and peak urinary flow rate (Qmax). Patients were followed post-procedure at foley removal (2-5 days), 30 days, 3 months, 6 months and yearly thereafter.
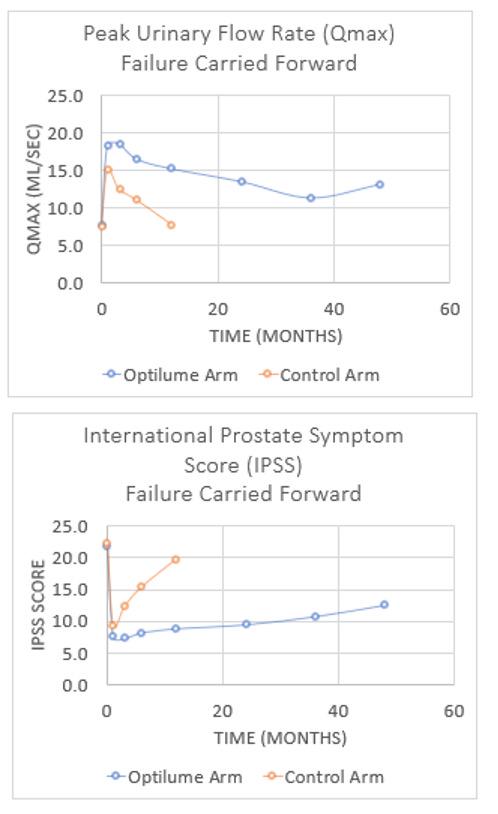
RESULTS: Subjects randomized to receive the DCB had an average of 3.2 prior treatments and an average stricture length of 1.6cm (46% ≥2cm), with 8/79 (10.1%) having penile strictures and 9/79 (11.4%) having received prior pelvic radiation. IPSS significantly improved from 21.9 at baseline to 12.6 at 4 years, which showed slight deterioration from the 3 year (11.3), 2 year (10.1) and 1 year (9.0) results. Qmax significantly improved from a baseline of 7.7 mL/sec to 13.2 mL/sec at 4 years, which is in line with the 3 year (12.0) and 2 year (13.9) data. Freedom from repeat intervention for DCB subjects was estimated to be 71%. No late-onset treatment related adverse events were observed.
CONCLUSIONS: The OptilumeŽ DCB continues to achieve significant improvements in symptoms, flow, and reintervention rates through 4 years post treatment.