BACKGROUND:
The transcorporal (TC) placement of artificial urinary sphincters (AUSs) may serve as a protective measure, especially in cases of fragile urethras. This study compares the lifespan of first-time TC AUSs versus standard AUSs in patients who have undergone prior radiotherapy (RT) for prostate cancer (PCa).
METHODS:
We conducted a retrospective review of patients who received AUS placements at two high-volume centers from January 1st, 2011, to July 1st, 2023. Inclusion criteria comprised men undergoing their initial AUS insertion (without prior AUS placement), with a history of PCa, and receiving RT, with or without radical prostatectomy (RP). Exclusion criteria included patients who underwent RT following AUS insertion or lacked data regarding the procedure approach (TC versus standard placements [SP]) or follow-up. Patients were categorized into two groups: TC and SP. The primary outcome assessed AUS lifespan by comparing the relative risk of device explantation and/or revision (Exp/Rev) between TC and SP groups. To mitigate potential bias arising from unequal follow-up periods between the two groups regarding Exp/Rev rates, a Kaplan-Meier curve was constructed to compare the 10-year lifespan timeframe of TC and SP. Secondary outcomes included investigating the causes of Exp/Rev, rates of device removal, reinsertions, and details of second device removal.
RESULTS:
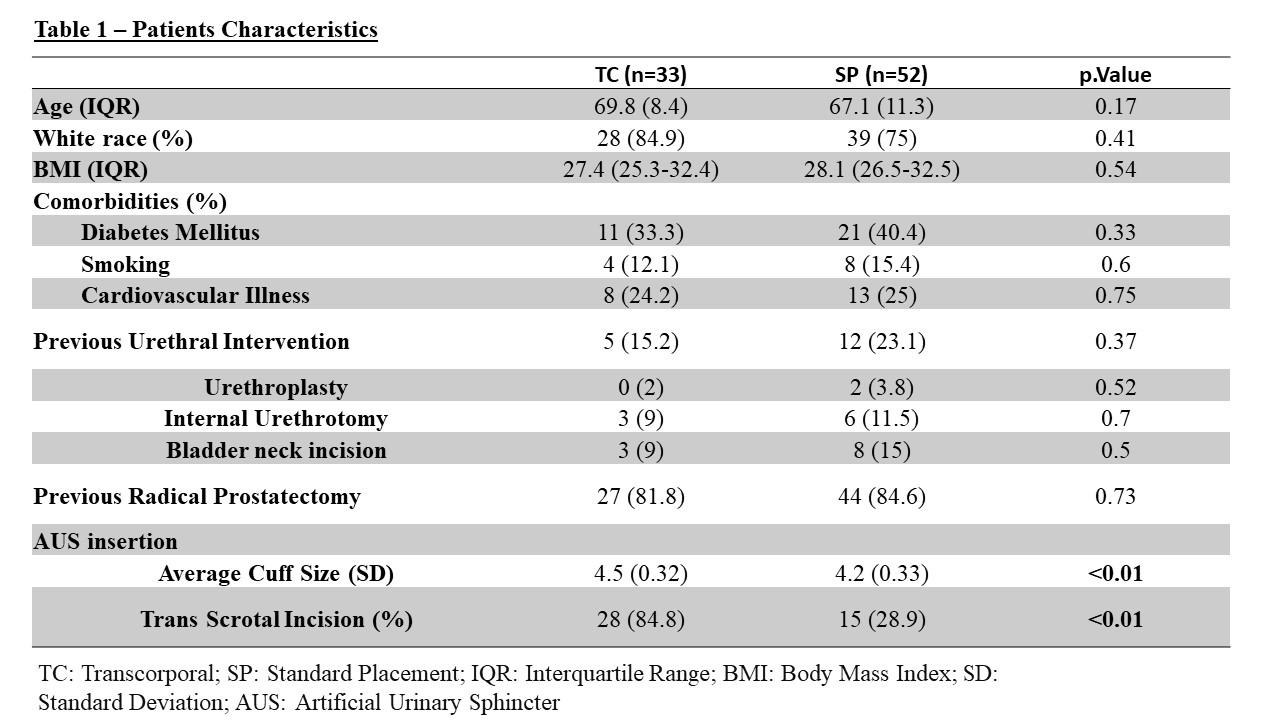
Out of 314 men with AUS, 85 met the inclusion criteria, comprising 38.8% (n=33) in the TC group and 61.2% (n=52) in the SP group. The median ages were 69.8 years (interquartile range [IQR] 65.2-73.6) for the TC group and 67.1 years (IQR 61.6-72.9) for the SP group, (p=0.17). Table 1 illustrates the characteristics of TC and SP patients.
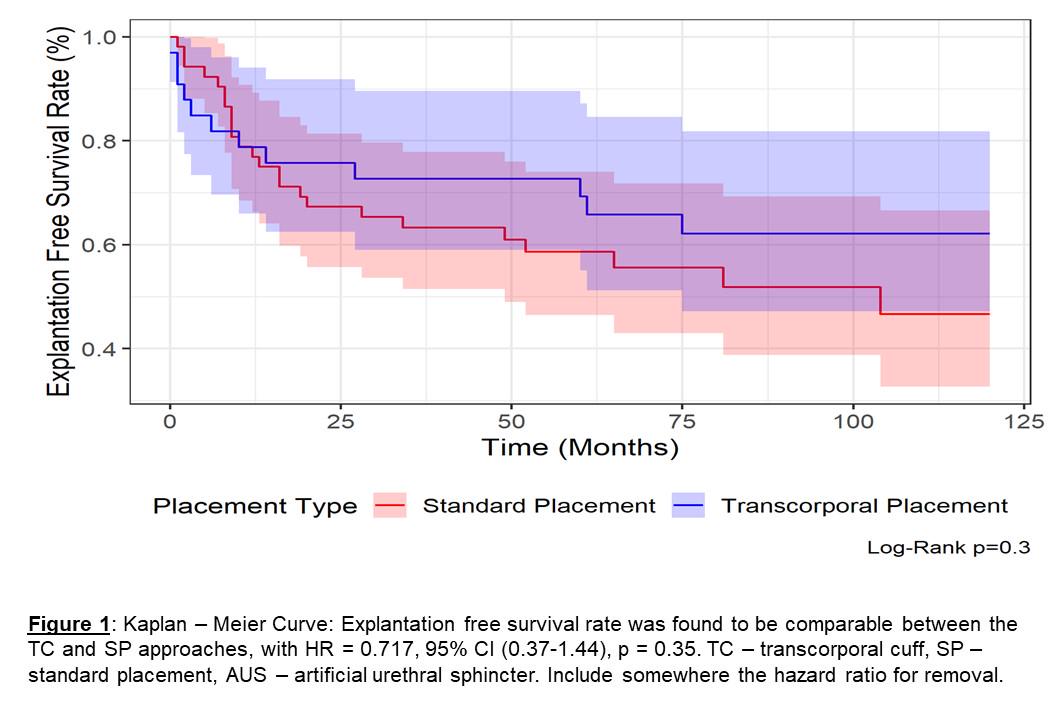
The median follow-up durations were 51.9 months (IQR 15.8-86.1) for the TC group and 80.4 months (IQR 28.1-128.3) for the SP group. During the follow-up period, 36.4% (12/33) of TC devices experienced Exp/Rev, with 12.1% (4/33) due to mechanical failures (MFs), 24.2% (8/33) due to erosions, and 6.1% (2/33) due to infections. In comparison, 55.8% (29/52) of SP devices underwent Rev/Exp, with 26.9% (14/52) due to MFs, 21.1% (11/52) due to erosions, and 9.6% (5/52) due to infections. However, no statistically significant differences were found between the two approaches, with a hazard ratio (HR) of 0.717 (95% confidence interval [CI] 0.37-1.44, p=0.35), (Figure1). The calculated device survival probabilities at one, five, and ten years were 78.8%, 69.3%, and 62.1% for the TC group, respectively, and 76.9%, 58.7%, and 46.7% for the SP group, respectively.
CONCLUSIONS:
TC cuff insertion for the first AUS implantation in pre-radiated patients showed to be comparable to SP when it comes to device survival, with comparable complication rates. Current guidance for approach selection is primarily based on patient selection and surgeon preference.