BACKGROUND: Ureteral stents are a common urologic intervention with nearly ninety-two thousand ureteral stents placed every year in the United States. Though commonly necessary, stents cause symptoms in many patients, impacting quality of life and posing considerable economic burden. The Tria Stent™ is a new product that employs material changes to allow the stent to soften at body temperature, purportedly making them more tolerable. This has not been evaluated in the clinical setting. Our goal is to compare patient experience with Boston Scientific Tria stents and more conventional Boston Scientific stents (e.g., Contour).
METHODS: Patients who received ureteral stents between 8/1/2023 and 2/1/2024 at our institution were reviewed. Inclusion criteria were bilateral or unilateral ureteral stent placement for endoscopic procedures related to nephrolithiasis, tumors, obstruction, or diagnostic ureteroscopy. Exclusion criteria were patients with stents placed for bladder/ureter surgery (e.g., cystectomy, ureterolysis, pyeloplasty). A block randomization strategy was employed where patients received Contour, Percuflex, or Polaris Ultra stents during the first 3-month period and Tria stents during the subsequent 3 months. Demographic data including age, gender, race/ethnicity, BMI, stent indication, stent dwell time (measured from day of procedure), stent width, patient height:stent length, ureteroscopy performed, operation time, pre-procedure stent, and positive urine culture were collected. Our primary endpoint was a binary composite of unplanned pain encounters. This was defined as ED/UC visits, clinic calls, Epic messages, and clinic/telemedicine visits for abdominal/flank pain, dysuria, urgency, or frequency. The secondary outcome, unplanned non-pain encounter defined as hematuria or urinary tract infection, was also assessed. Univariate analysis between Tria and non-Tria stents was performed on baseline variables using chi-square test or Fisher’s exact test for categorical variables and student’s t-test or Mann-Whitney test for continuous variables. A multivariate logistic regression model demonstrates the association between stent type (Tria vs. non-Tria) and our primary outcome, controlling for baseline characteristics (Table 2). Significance was determined using a P-value cutoff of 0.05.
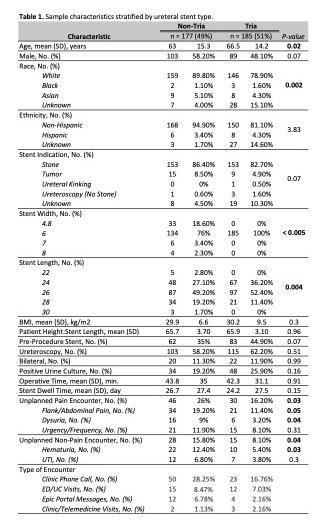
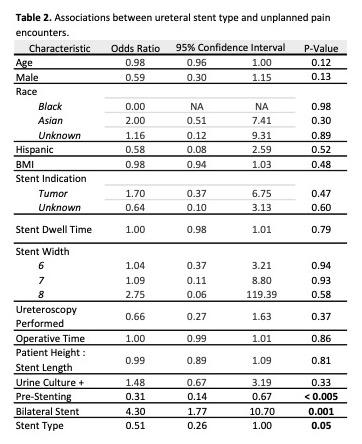
RESULTS: 362 stents were placed during the study period: 185 Tria stents (51%), 139 Contour (38%), 35 Percuflex (10%), 3 Polaris Ultra (1%). On unadjusted analysis, patients with Tria stents were less likely than non-Tria stents to have an unplanned pain encounter (16% vs. 26%, respectively; p = 0.03) and unplanned non-pain encounter (8% vs. 16%, respectively; p = 0.035). Multivariate logistic regression demonstrated a lower odds of unplanned pain encounter with use of Tria stents (OR = 0.51; p = 0.05) and pre-procedure stenting (OR = 0.31; p < 0.005). Bilateral stent placement was associated with more unplanned pain encounters (OR 4.3; p < 0.005).
CONCLUSIONS: Tria stents result in decreased unplanned post-procedure encounters for stent pain symptoms. As such, Tria stents may contribute to improved patient satisfaction and decreased healthcare costs.