Safety and efficacy of Stereotactic MRI-Guided Adaptive Radiation Therapy for Localized Kidney Tumors
Kendrick Yim, MD1, Daniel Cagney, MD1, Raymond Mak, MD1, Lisa Singer, MD1, Christopher Williams, MD1, Vincent D'andrea, MD1, Neil Martin, MD1, Toni Choueiri, NMD2, Steven Chang, MD1, Jonathan Leeman, MD1.
1Brigham and Women's Hospital, Boston, MA, USA, 2Dana Farber Cancer Institute, Boston, MA, USA.
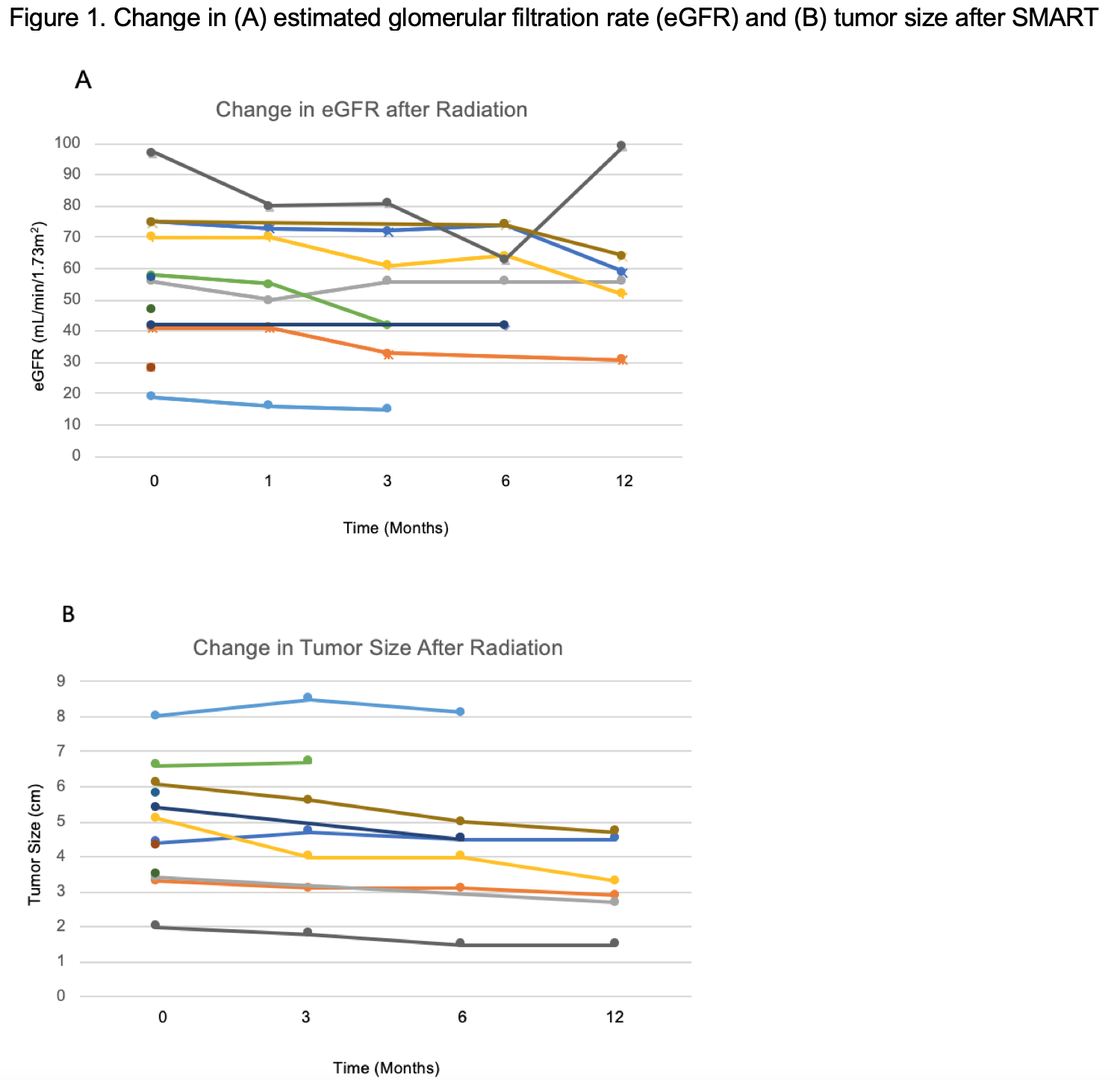
BACKGROUND: To assess the safety, feasibility as well as short term oncologic and renal functional outcomes of stereotactic MRI guided adaptive radiotherapy (SMART) for localized kidney cancer in non-surgical candidates. METHODS: Single institution experience of patients undergoing MRI guided radiation therapy with daily plan adaptation for localized kidney cancer from 2020-2021. Patients were treated to a dose of 4000cGy over 5 fractions. Prior to each fraction, a pre-treatment MRI was obtained to allow for re-contouring of tumor and organs-at-risk. Patients were treated using an MR guided gated breath hold technique. Adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) guideline version 5.0. RESULTS: Our cohort includes 12 patients with median age 75 years, median tumor diameter 4.75 cm, and median RENAL Nephrometry score 7. In terms of staging, 5 were cT1a, 5 were cT1b, 1 was cT2a, and 1 was cT3a. In total, 67.7% of patients had baseline Stage III CKD or worse renal function, and median Charlson Comorbidity index was 8. Histologic subtypes were 58.3% RCC, 25% papillary RCC, and 16.7% not reported. There was a total of 11 adverse events (AE) within 90 days: 10 were grade 1 for fatigue, nausea, vomiting, and diarrhea; there was one grade 2 AE for nausea (Table 1). No grade 3 or higher events were observed. The median baseline eGFR was 56.5 mL/min/1.73m2, with median change in eGFR of -10 mL/min/1.73m2 and the median change in tumor diameter was -0.5 cm at 7.5 months (IQR 2.25 - 12.25 months) of follow-up, with no local or distant progression. No SMART-related hospital admissions occurred (Figure 1). CONCLUSIONS: SMART is a safe outpatient treatment option with low toxicity, limited decline in renal function, and high rates of local tumor control. For patients who are not surgical candidates, SMART may be an effective therapy for localized kidney cancer, especially for those less suitable for ablative therapies due to larger tumor size, close proximity to the collecting system, and use of anticoagulation. 
Back to 2022 Abstracts


