Risky Business; The Use of Unregulated Phosphodiesterase Inhibitors in Healthcare Supplements
Rachel M. Greenberg, Joseph Caputo, Gabriella Avellino, Mark Sigman, Elias Hyams
Rhode Island Hospital, Providence, RI, USA.
BACKGROUND: Sildenafil and tadalafil are two Food and Drug Administration (FDA)-approved prescription drugs used to treat erectile dysfunction (ED). Though highly effective in the management of ED, they can cause adverse reactions due to their vasodilatory effects, and are contraindicated in men on nitrates. Lack of regulation of dose amounts can increase the risk of significant side effects. Additionally, the metabolism of PDE5i may be affected by other medications, leading to potential toxicity. In this study, we investigated reports of PDE5i in healthcare supplements unregulated by the FDA to characterize unmonitored exposure to these medications in the community.
METHODS: The publicly available Food and Drug Administration's (FDA) “Health Fraud Data Base” (images/g66_1.png" target="_blank">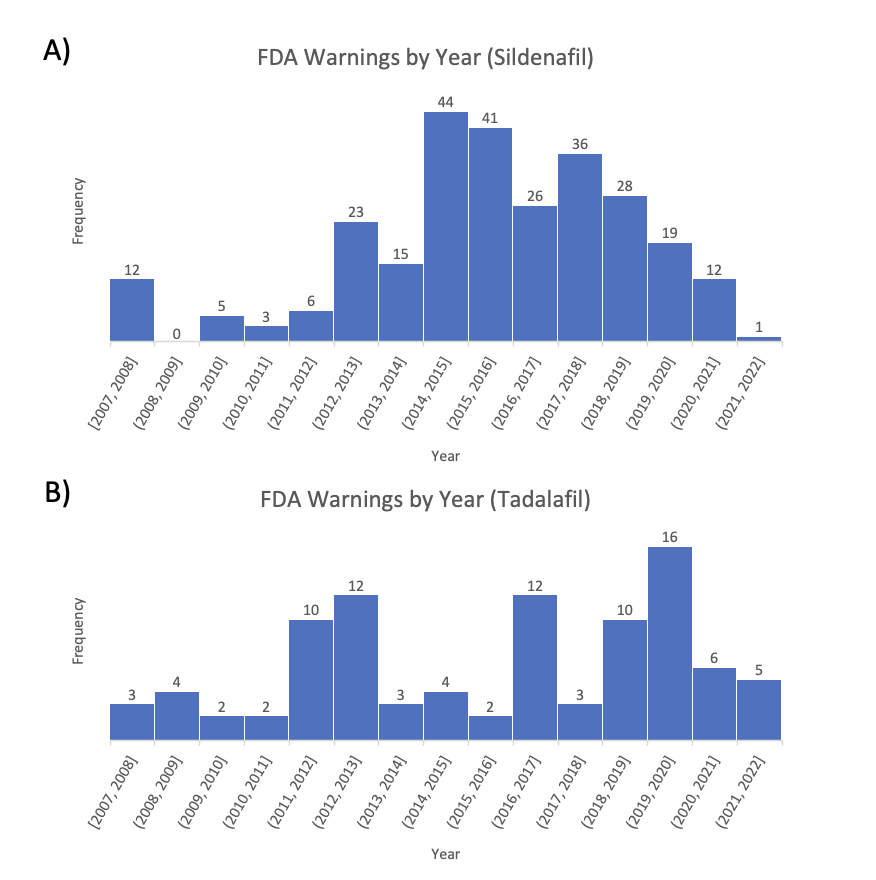
Back to 2022 Abstracts


