Impact of family history and germline genetic risk variants on long-term outcomes of active surveillance-eligible prostate cancer
Florian Rumpf, BS1, Anna Plym, PhD2, Jane B. Vaselkiv, MPH2, Mark A. Preston, MD, MPH3, Adam S. Kibel, MD3, Lorelei Mucci, ScD2, Keyan Salari, MD, PhD1.
1Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA, 2Harvard T.H. Chan School of Public Health, Boston, MA, USA, 3Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
BACKGROUND: A family history of prostate cancer (PCa) and BRCA/DNA repair gene-associated cancers are risk factors for developing prostate cancer. In the last decade, >260 PCa-associated single nucleotide polymorphisms (SNPs) have been identified through large-scale genome-wide association studies that strongly stratify lifetime risk of prostate cancer. Few studies have examined the prognostic value of family history or PCa germline risk variants in patients with favorable-risk PCa eligible for active surveillance (AS). Here, we evaluate the impact of a positive family history and germline risk SNPs on outcomes on AS-eligible patients.
METHODS: In a prospective cohort of 39,427 U.S. men in the Health Professionals Follow-up Study, 1,367 were diagnosed with clinically favorable-risk (NCCN very low-, low-, or favorable intermediate-risk) PCa from 1986 to 2016 and underwent genome-wide SNP genotyping. During the 30-year follow-up, 263 patients experienced disease recurrence and 52 patients died of PCa. A polygenic risk score was derived from 269 SNPs associated with development of PCa. Two loci associated with PCa survival were also assessed. Multivariable Cox proportional hazards models were used to estimate the association between family history of prostate, breast, or pancreatic cancer, germline risk SNPs, and PCa recurrence or death through 2019.
RESULTS: The median follow up time from diagnosis was 14.1 years. A family history of prostate, breast, or pancreatic cancer was observed in 494 (36%) patients. Men with a positive family history had an 83% greater risk of PCa-specific death (95% CI 1.06-3.17, p=0.03), and greater than that of men with a family history of PCa alone. The polygenic risk score was not associated with either PCa recurrence or death. However, focusing on 2 loci on 8q24 and 19q13 with the strongest evidence in the literature for association with PCa aggressiveness, we observed a significant association between rs2735839 on 19q13 and PCa-specific death (HR 1.81, 95% CI 1.04-3.17, p=0.037). Using a risk score giving one point each to a positive family history and rs2735839 risk allele, each point of the heritable risk score was associated with a 72% increased risk of PCa-specific death (95% CI 1.16-2.54, p=0.006).
CONCLUSIONS: Our study found that patients with AS-eligible PCa with a family history of prostate, breast, or pancreatic cancer or a 19q13 germline risk allele had elevated risks of PCa-specific death. These findings have implications for how germline genetic risk should be factored into AS selection criteria. 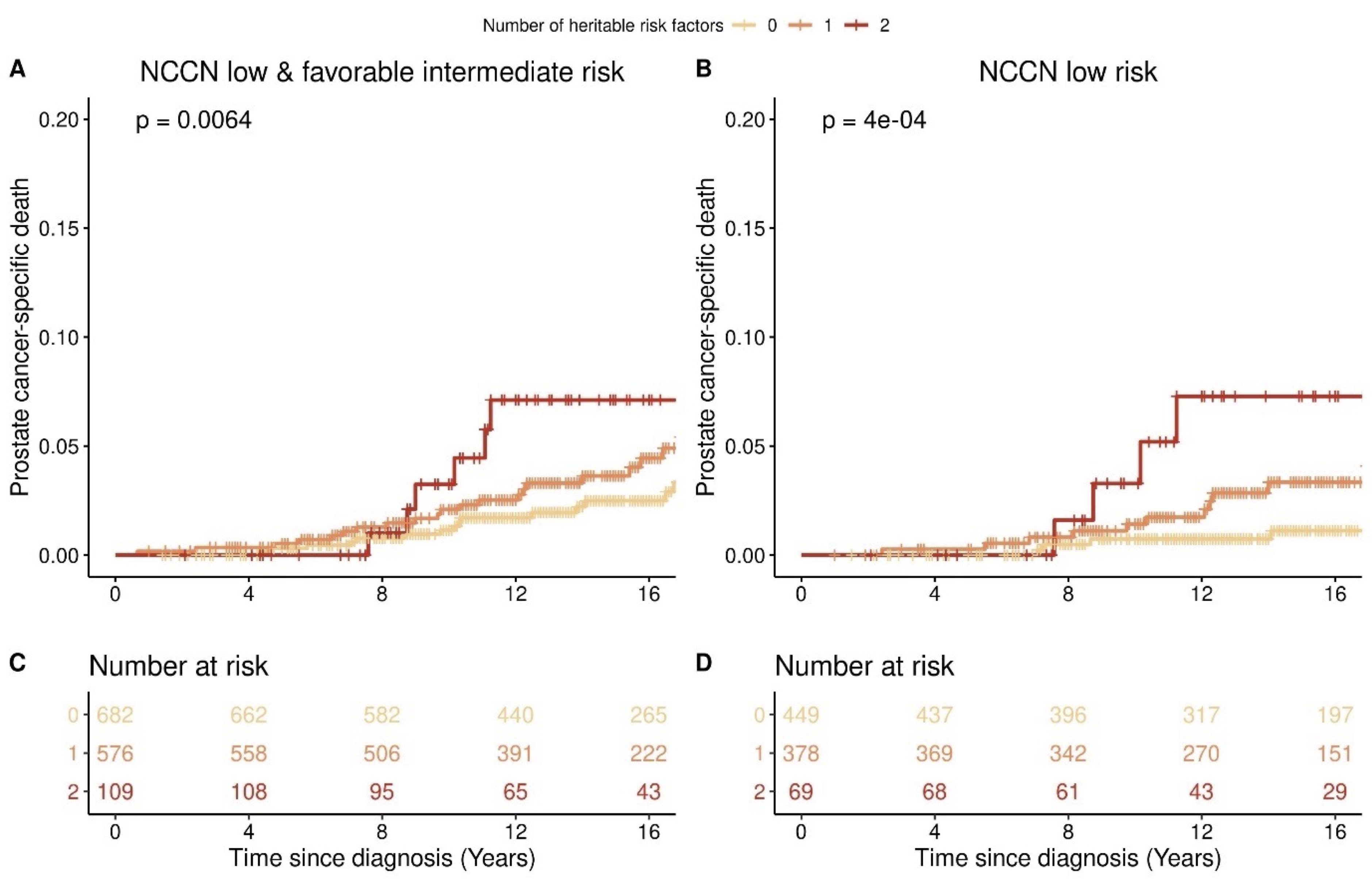
Back to 2022 Abstracts


