Vibegron Shows Meaningful Changes in Clinical Endpoints in Patients with Overactive Bladder: Analyses From EMPOWUR
Michael Kennelly, MD1, Cynthia J. Girman, DrPH, FISPE2, Jeffrey Frankel, MD3, David Staskin, MD4, Susann Varano, MD5, Matt T. Rosenberg, MD6, Diane K. Newman, DNP, ANP-BC7, Denise Shortino, MS8, Rachael A. Jankowich, RN, MSN8, Paul N. Mudd, Jr., PharmD, MBA8.
1Carolinas Medical Center, Charlotte, NC, USA, 2CERobs Consulting, LLC, Chapel Hill, NC, USA, 3Seattle Urology Research Center, Seattle, WA, USA, 4Tufts University School of Medicine, Boston, MA, USA, 5Clinical Research Consulting, Milford, CT, USA, 6Mid-Michigan Health Centers, Jackson, MI, USA, 7Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA, 8Urovant Sciences, Irvine, CA, USA.
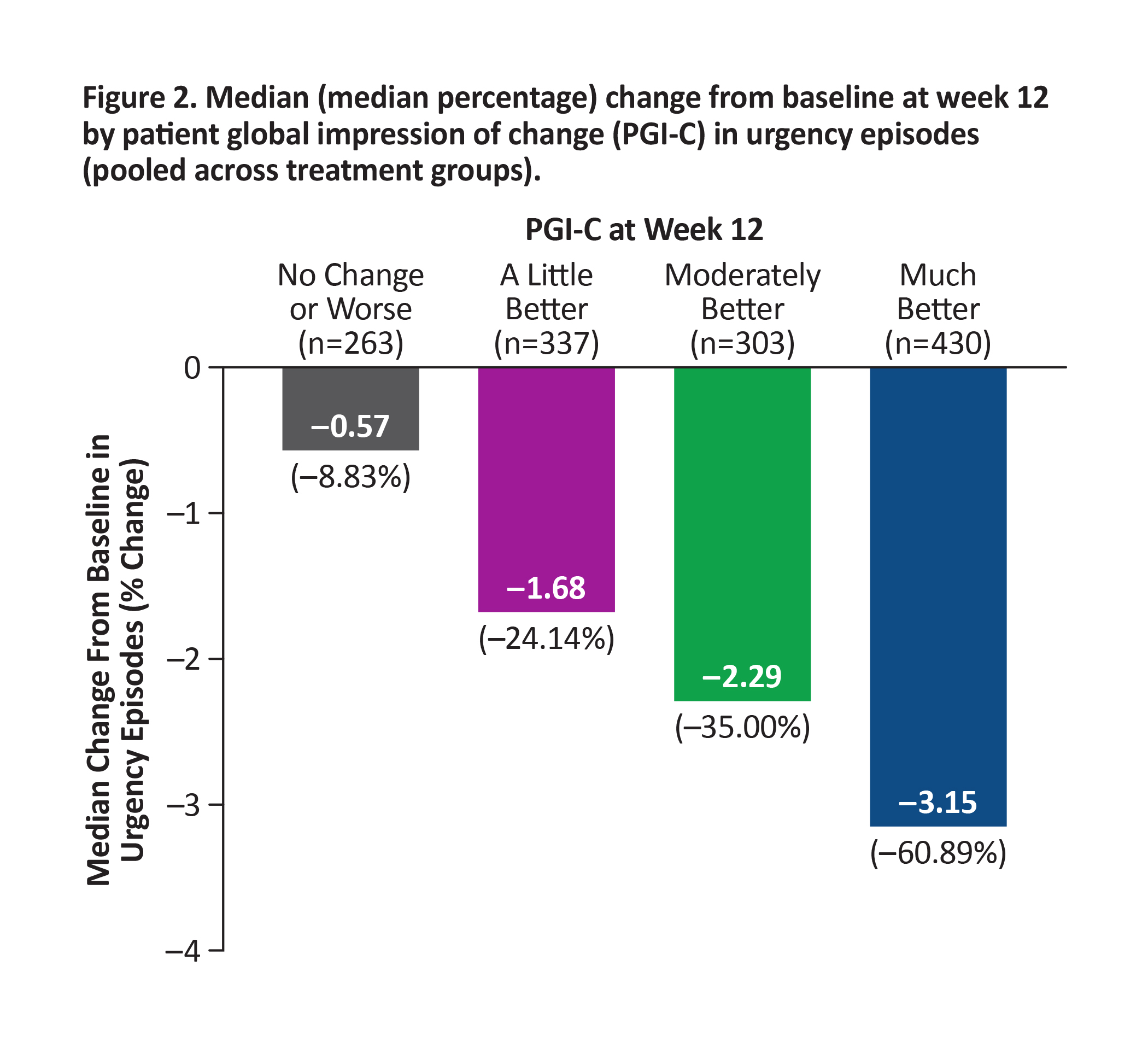
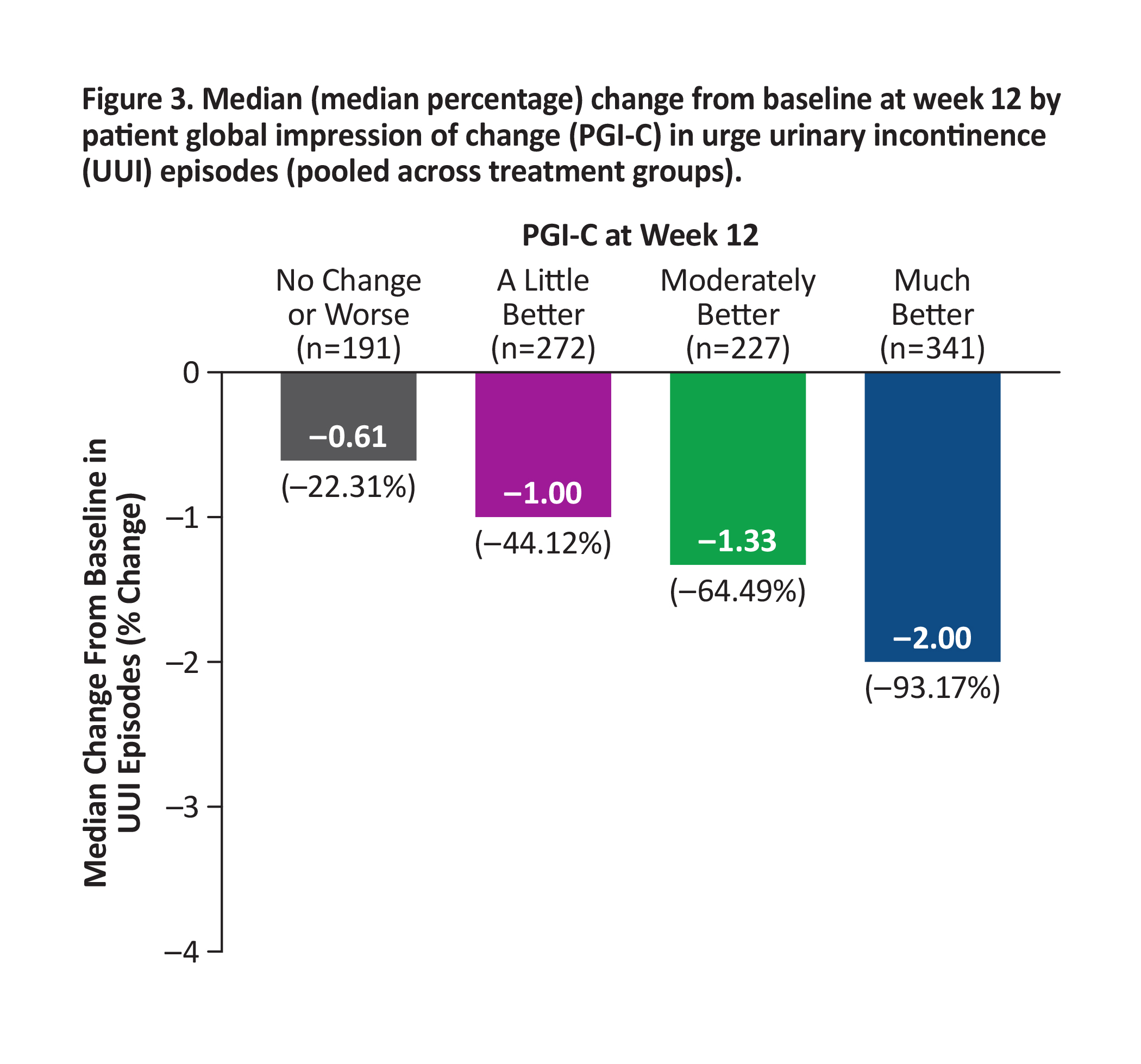
BACKGROUND: Reductions in bothersome symptoms of overactive bladder (OAB) are reported to demonstrate improvement in clinical trials, often without interpreting meaningfulness to patients. In the 12-week phase 3 EMPOWUR trial, vibegron significantly reduced micturitions, urgency episodes, and urge urinary incontinence (UUI) episodes vs placebo (P<0.01, each). These analyses used an anchor-based approach to interpret the meaningfulness of symptom reduction with the patient global impression of change (PGI-C).
METHODS: Median change from baseline (CFB) at week 12 in micturitions, urgency episodes, and UUI episodes was generated for each PGI-C category, pooled across treatments. Percentages of patients achieving these reductions in symptoms were determined.
RESULTS: Patients who experienced greater CFB at week 12 in each endpoint reported greater improvement in PGI-C. Median reductions from baseline in OAB endpoints pooled across treatment groups were higher than thresholds patients perceived as improved based on PGI-C: ≥15% reduction in micturitions (moderately better; Figure 1), ≥50% reduction in urgency episodes (much better; Figure 2), and ≥90% reduction in UUI episodes (much better; similar to ≥75% reduction seen in EMPOWUR [PGI-C: moderately better]; Figure 3). Significantly more patients receiving vibegron vs placebo achieved these reductions (P<0.05, each).
CONCLUSIONS: Significantly more patients treated with vibegron vs placebo in EMPOWUR achieved meaningful reductions in micturitions and urgency/UUI episodes that were associated with patient-perceived improvement. 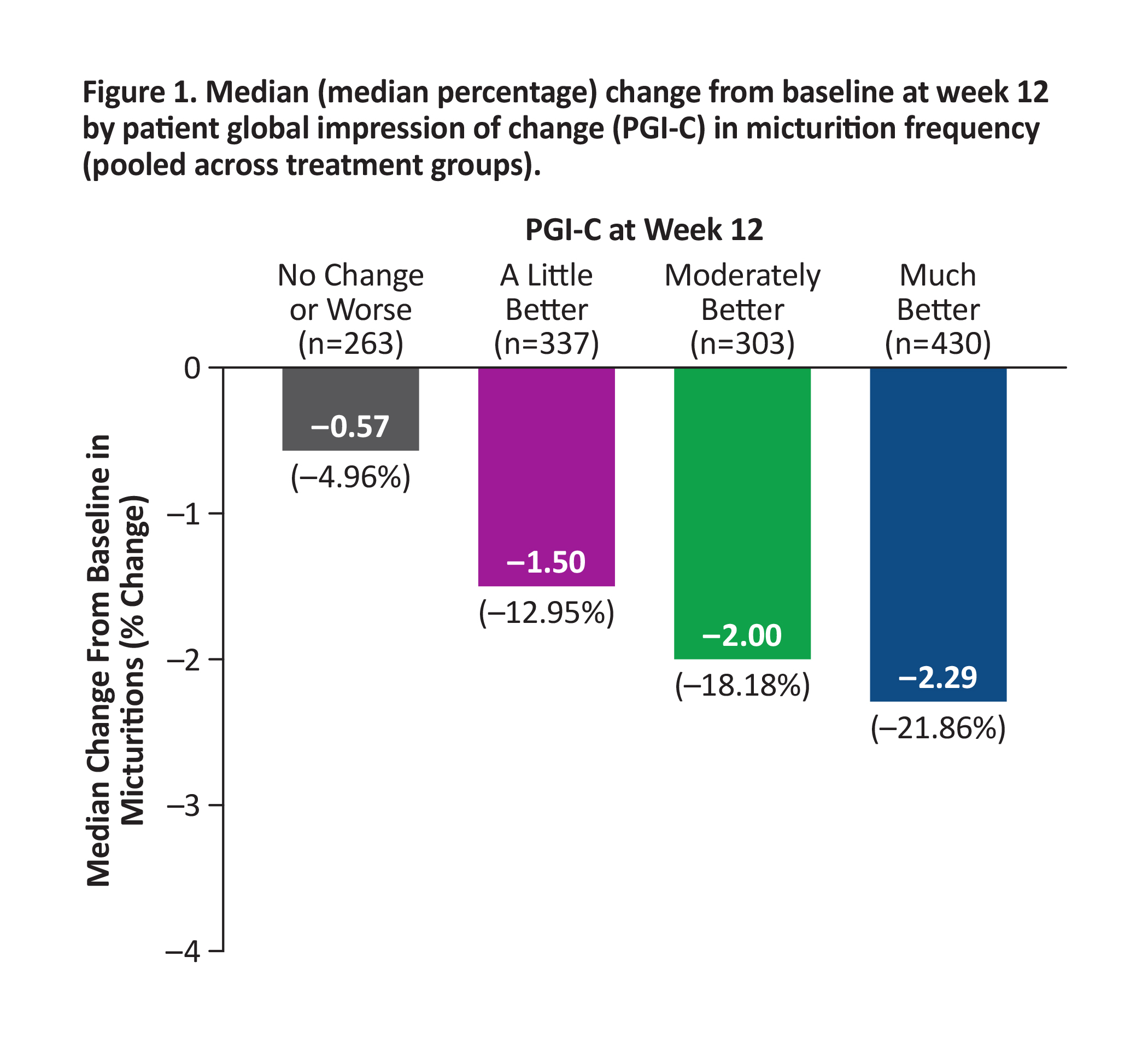
Back to 2021 Abstracts
