The Association Between the 17-gene Genomic Prostate Score Result and Active Surveillance, Therapy Intensity and Complications in NCCN Favorable Intermediate-Risk Prostate Cancer Patients
Christopher Pieczonka, MD1, Benjamin Lowentritt, MD, FACS2, Eric Margolis, MD3, Edward Uchio, MD, FACS, CPI4, Marina Pavlova, MS5, Kenny Wong, BS5, John Bennett, PhD5, Melissa Stoppler, MD6.
1Associated Medical Professionals of NY, Syracuse, NY, USA, 2Chesapeake Urology, Towson, MD, USA, 3New Jersey Urology, Englewood, NJ, USA, 4University of California, Irvine, Irvine, CA, USA, 5Exact Sciences, Pacific Grove, CA, USA, 6Exact Sciences, Redwood City, CA, USA.
BACKGROUND: This study determined how the 17-gene Genomic Prostate Score® (GPSTM) molecular assay guides treatment decisions in patients with NCCN favorable intermediate-risk (FIR) prostate cancer (PCa).
METHODS: This retrospective study included 324 patients from 7 urology practices in the United States. All had NCCN FIR PCa, defined as <50% positive biopsy cores and only one of the following risk factors: grade group 2 (Gleason Score 3+4), clinical stage T2b-T2c, or PSA 10-20 ng/mL. All had a GPS assay report dated May 2017 to April 2019. The GPS assay reports a result between 0 and 100, with higher values associated with higher likelihood of adverse pathology and higher risk of distant metastasis and PCa-specific mortality. Data were collected from patients' charts/electronic health records. The proportions who selected active surveillance (AS) and definitive treatment with monotherapy or multimodal therapy were calculated with 95% confidence intervals (CI) using the Clopper-Pearson method. In addition, the association of several clinical variables, including Gleason Score, PSA, and number of positive cores, and the GPS result with AS selection was evaluated in uni- and multivariable logistic regression models.
RESULTS: Among the 324 patients, 79% had grade group 2 tumors, 19% had PSA 10-20 ng/mL, and 2% were clinical stage T2b. Median follow-up time was 18 months, and one PCa-related metastasis and 2 deaths (neither PCa-related) were reported. For the GPS assay, 76 patients had results <20, 195 had results between 20 and 40, and 53 had results >40. Overall, 31% (95% CI 26%, 36%) selected AS. Gleason Score, PSA, number of positive cores and GPS result were all significantly associated with AS selection in univariable logistic regression models and number of positive cores and GPS result remained significant in multivariable models including these four variables. AS percentage decreased as GPS results increased: 58% (95% CI 46%, 69%) selected AS with GPS results 0-19, 27% (95% CI 21%, 33%) with GPS results 20-40, and 6% (95% CI 1%, 16%) with GPS results 41-100. AS percentage was lower for patients with more positive cores: 39% (95% CI 31%, 46%) of patients with 1-2 positive cores selected AS and 22% (95% CI 15%, 29%) with 3 or more positive cores. Patients with GPS results 0-19 and 20-40 were more likely to receive monotherapy than with GPS results >40. Patients with GPS results >40 were more likely to receive multimodal therapy (Figure 1). Complications (erectile dysfunction, urinary/bowel incontinence) were more common in treated than AS patients. AS persistence was 91% (95% CI 82%, 95%) at 12 months; patients discontinued AS due to disease progression (61%) or patient preference/unknown reasons (39%).
CONCLUSIONS: The GPS result appears to be associated with selection of AS and treatment intensity in this first examination of a genomic classifier in a cohort of NCCN FIR PCa patients. 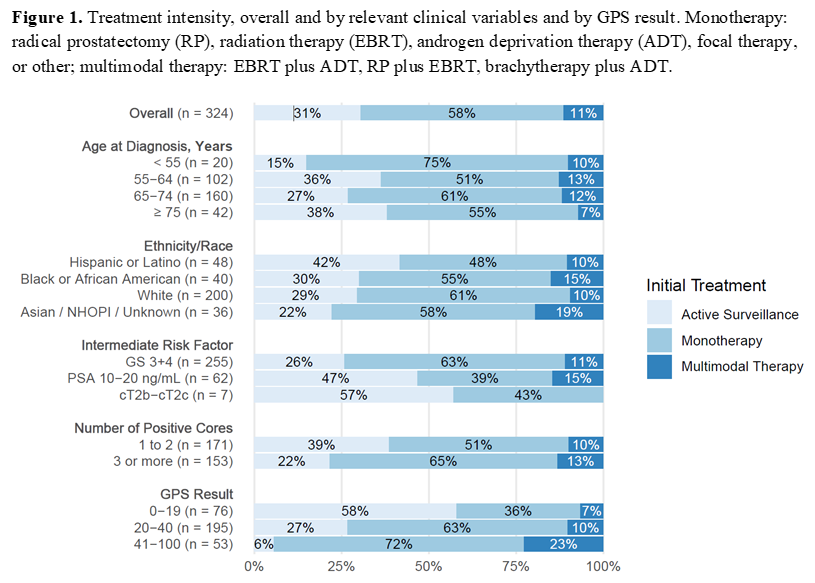
Back to 2021 Abstracts
