Premature Termination of Genitourinary Cancer Trials: Assessing Trial Efficiency Using Novel Algorithms
Kristian Stensland, MD, MPH1, Krystal DePorto, BS2, James Ryan, BS2, Matthew Galsky, MD3
1Lahey Hospital and Medical Center, Burlington, MA, 2Tufts University School of Medicine, Boston, MA, 3Icahn School of Medicine at Mount Sinai, New York, NY
BACKGROUND: Cancer clinical trials fail to reach their planned primary endpoint at a reportedly high rate. Trials which fail prematurely do not contribute maximally to the knowledgebase, if at all, and divert patients from other trials. Optimizing the conduct of cancer clinical trials through improved trial planning, and identifying trials at higher risk of failing to complete, could streamline the trials enterprise and hasten the investigation of new treatments while minimizing patient and investigator burdens. We identified associations with premature termination in genitourinary cancer trials using novel data extraction algorithms. METHODS: We extracted clinical trial data from ClinicalTrials.gov for prostate, bladder, kidney, testicular, and ureteral cancers. We included only Phase 2-3 interventional trials started in 2007 or later that had completed or terminated. We designed data extraction algorithms to generate previously unavailable data points for trials, including sponsor and trial information, anticipated and actual accrual numbers (method previously validated and published), and site number and location. We then manually coded reasons for premature termination from the provided free text in the trial record. We considered "toxicity," "adverse events," or "interim analysis" to be appropriate reasons for trial termination as these reasons provide useful information. We identified associations with premature termination via a logistic regression model, with covariates as detailed in Table 1. RESULTS: A total of 747 trials were included. Of these, 231 (30.9%) terminated early, and 193 (25.8%) terminated for a reason other than toxicity/efficacy. The most common reason for termination was poor accrual (43.3%). On multivariable logistic regression, trials with sites outside the USA and prostate cancer trials were less likely to prematurely terminate. Of trials reported as "completed," 84% met at least 75% of their anticipated accrual goal. CONCLUSIONS: The rate of premature termination in genitourinary cancer trials is high, with more than 1 in 4 trials terminating prematurely for reasons other than toxicity or efficacy. Interventions are direly needed to optimize clinical trial conduct in order to decrease the drain on patient and investigator resources and hasten much-needed advances in genitourinary cancer care. 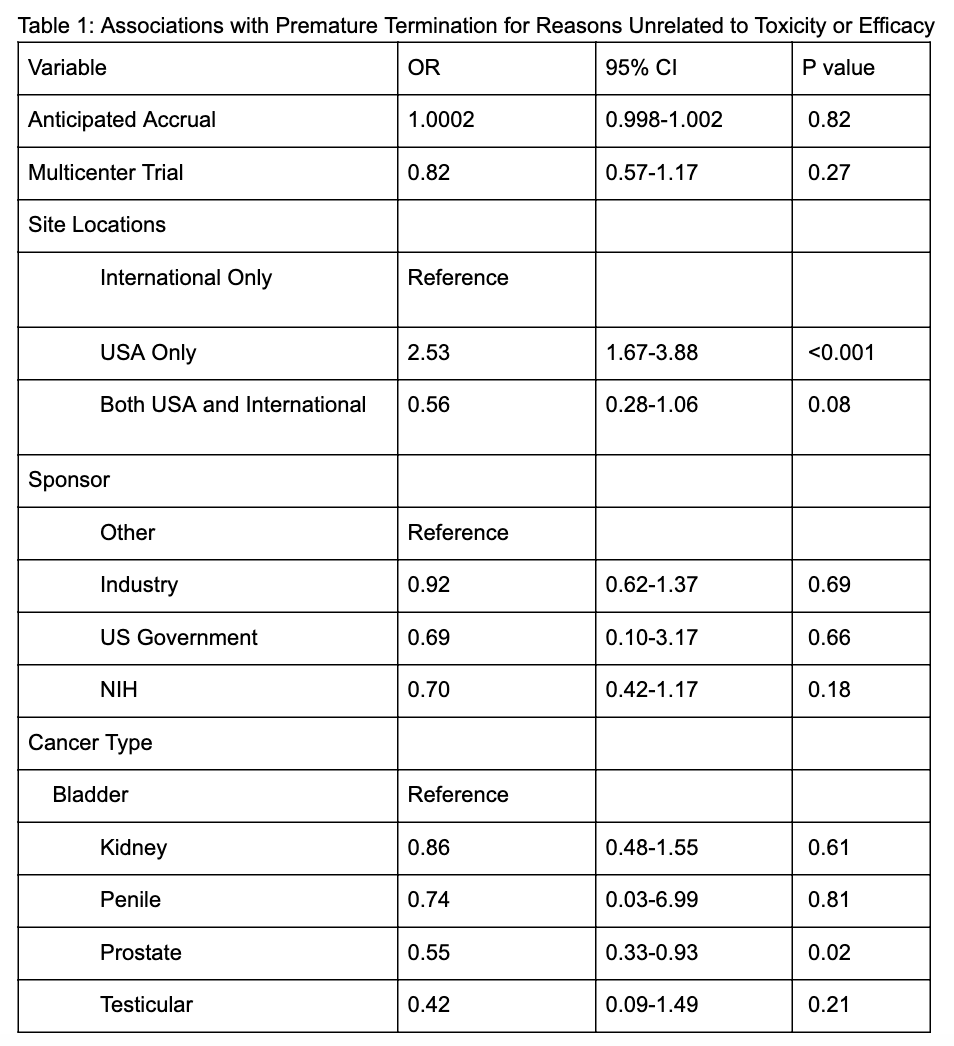
Back to 2019 Abstracts
