Independent Contributions of MRI Fusion and Systematic Prostate Biopsies to Detect Clinically Significant Prostate Cancer: A Multi-Institutional Review
Dylan M. Buller, BA1, Guy J. Manetti, MD2, Brandon C. Stahl, MD3, Joseph R. Wagner, MD4
1University of Connecticut School of Medicine, Farmington, CT, 2Urology Associates of Danbury, PC, Danbury, CT, 3Hartford Healthcare, William Backus Hospital, Norwich, CT, 4Hartford Healthcare, Hartford, CT
BACKGROUND: MRI fusion biopsies are being increasingly utilized to both diagnose and manage prostate cancer. However, the added benefit of fusion biopsies compared to systematic biopsies varies greatly in the literature. We compare the clinically significant cancer detection rates (≥ Grade Group 2 (GG 2)) between 3 separate institutions (A,B,C) performing concurrent targeted and systematic biopsies.
METHODS: MRI fusion biopsies performed at each institution over similar time periods were identified. Fusion biopsies were performed with the UroNav system at two institutions (A,C) and the BioJet system at the other. Patient demographics, clinical information, MRI/TRUS results, and pathology results were collected. Chi square comparisons of proportions were used for statistical analysis (MedCalc).
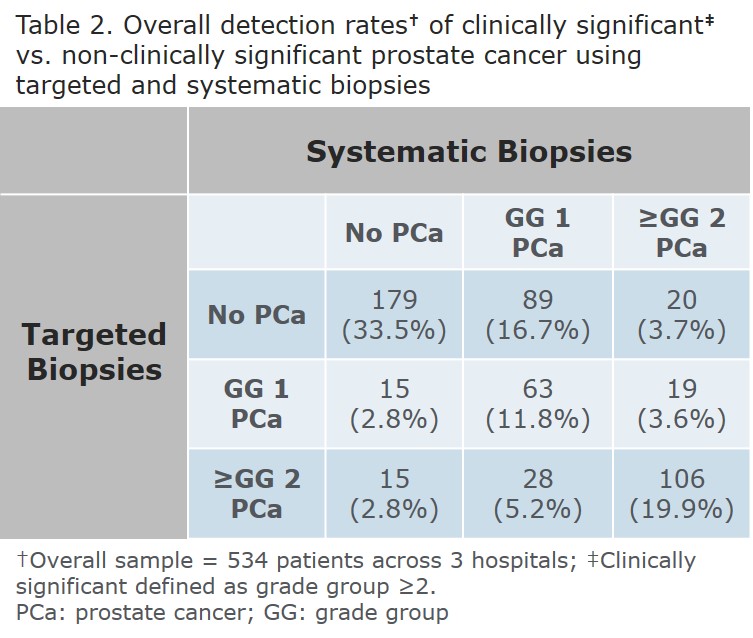
RESULTS: 256, 76, and 202 men underwent MRI fusion biopsies at institutions A, B, and C respectively with demographics and overall cancer detection rates as shown (Table 1). There were more men on active surveillance at Institution A (p<0.0001); age at biopsy, PSA, number of cores obtained, and cancer detection rates were similar across institutions. The rate of ≥GG 2 prostate cancer detected by targeted biopsies but missed by systematic biopsies was 5.5%, 18.4%, and 7.4% at institutions A, B, and C with an overall rate of 8.0%. The rate of systematic biopsies detecting ≥GG 2 prostate cancer missed by targeted biopsies was 9.8%, 2.6%, and 7.0% with an overall rate of 7.3% (Table 2). Using the presence of ≥GG 2 cancer on any biopsy as the presumed incidence, the sensitivity of targeted biopsies to detect ≥GG 2 cancer is 66.2%, 93.75%, and 85.4% respectively. Targeted biopsies are statistically less sensitive at Institution A v B (p=0.006) and A v C (p=0.009) but similar at B and C (p=0.370). The sensitivity of random biopsies to detect ≥GG 2 cancers is 81.1%, 56.25%, and 81.7%. Random biopsies were statistically more sensitive at Institution A v B (p=0.015) and C v B (p=0.010) but similar at A and C (p=0.910). Comparing the sensitivity of random and targeted biopsies within each institution demonstrates only Institution B has a higher detection rate with targeted biopsies (p=0.002). Institution A approached statistical significance in favor of random biopsies (p=0.061), and there was no difference at Institution C (p=0.700).
CONCLUSIONS: The value of targeted and systematic biopsies to detect ≥GG 2 prostate cancers varied widely between 3 institutions. Limits of this study include its retrospective nature, an unequal distribution of total patients, an unequal distribution of patients with prior prostate cancer, equipment variability, and user variability. It stresses the importance of all institutions to critically examine their own outcomes. Overall, MRI fusion biopsies uniquely identified 8.0% of ≥GG 2 prostate cancers while systematic biopsies uniquely identified 7.3% indicating that both methods significantly contribute to risk stratification. 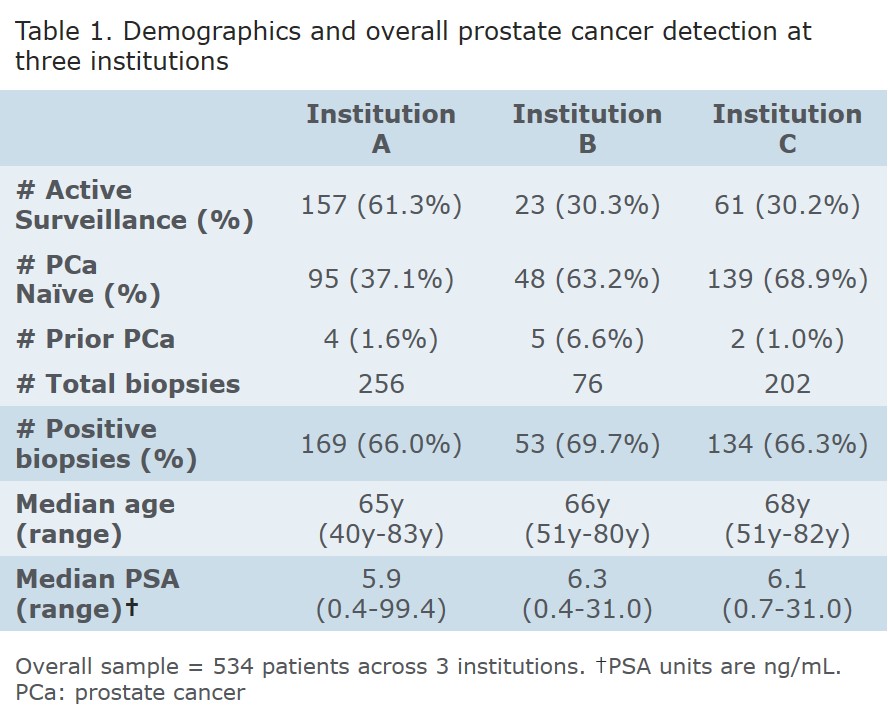
Back to 2019 Abstracts
