Back to 2017 Program
The Impact of the FDA Testosterone Supplementation Therapy Safety Advisory on Prescribing Patterns
Valary T. Raup, MD1, Tyler McClintock, MD1, Allen Seftel, MD2, Martin Kathrins, MD1.
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA, 2Cooper University Hospital, Camden, NJ, USA.
BACKGROUND: On March 3, 2015 the Food and Drug Administration (FDA) released a safety advisory and prescription labeling change which warned of possible cardiovascular side effects of testosterone supplementation therapy (TST). We sought to investigate the effect of the warning on TST prescribing patterns in the Boston metro-area.
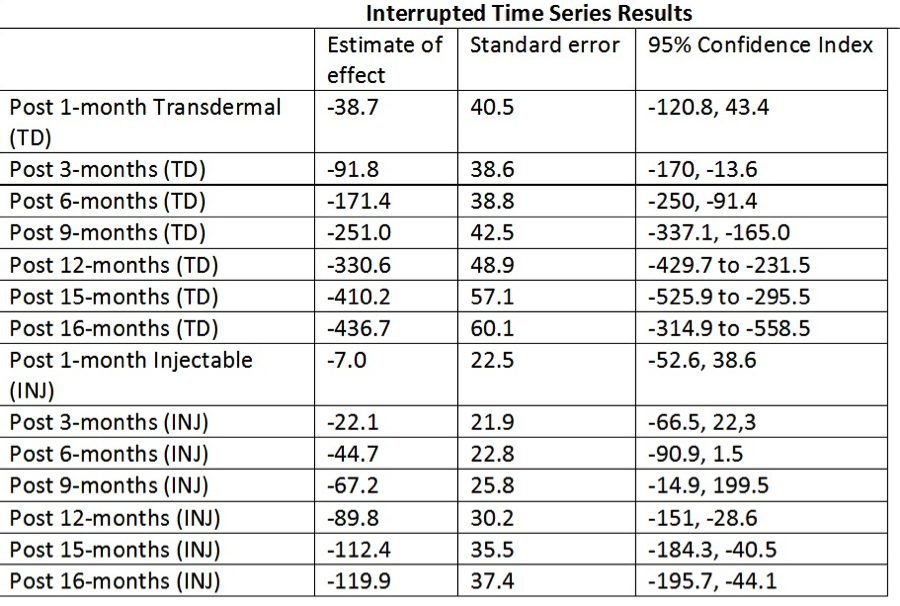
METHODS: We utilized the Research Patient Data Registry, which is a de-identified clinical patient data repository pertaining to eight hospitals in the Boston metro area within Partners Healthcare. We queried the database for men age 45 to 84 years old who were prescribed TST with transdermal or injectable formulations from March 2013 until August 2016. A second query was performed of such patients to quantify the monthly instances in which during which primary diagnosis of hypogonadism was recorded (based on ICD9/10 codes). We three performed separate interrupted time series (ITS) analyses for each TST modality type and monthly visits for hypogonadism, all with respect to the March 2015 safety advisory. We calculated estimates of effect and relative effects for 1, 3, 6, 9, 12, 15, 16 months post-safety advisory. All data analyses were performed with SPSS v20. IRB approval was not necessary due to the de-identified nature of the data.
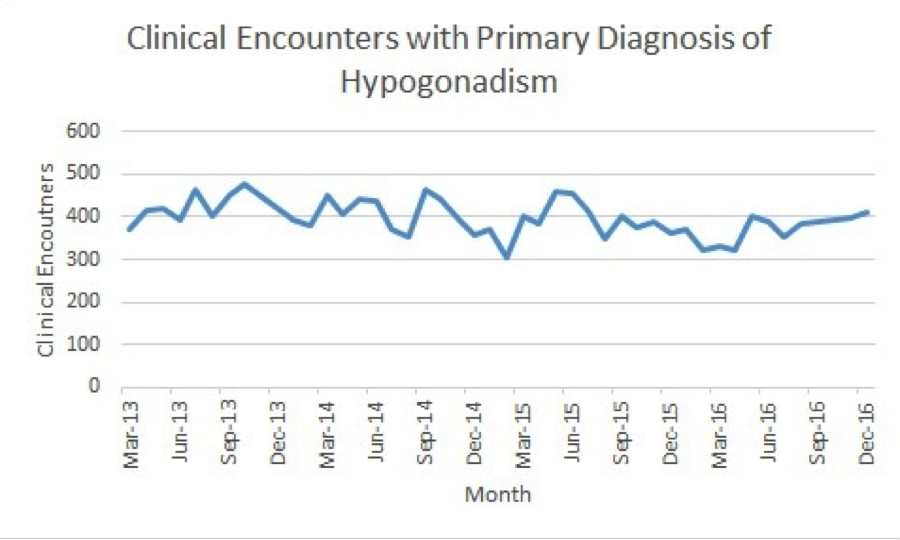
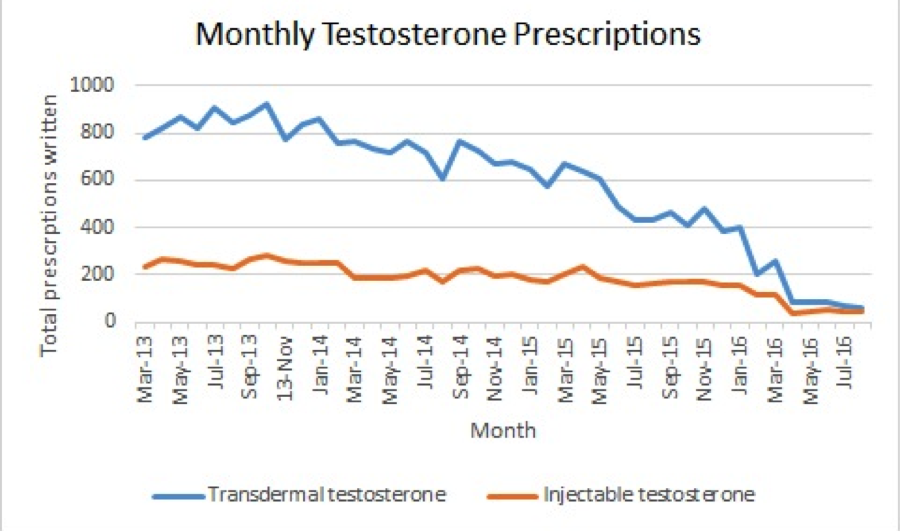
RESULTS: The monthly prescription quantities and clinical encounters are demonstrated in Figures 1 and 2, respectively. The ITS analysis revealed the difference in slope coefficients between pre- and post-safety advisory was -26.5 (p=0.000) for transdermal formulations. The difference in coefficients was -7.5 (p=0.003) for injectable formulations. However, the difference in coefficients for clinical encounters was -3.0 (p=0.260). The monthly interval estimate of effect and the monthly interval relative effect on prescription (when compared to expected monthly prescription rates based on the pre-safety advisory coefficient) are listed in Table 1. Limitations of the study were the inability to distinguish between new and renewed prescriptions and the retrospective nature of the database.
CONCLUSIONS: While the monthly number of interactions resulting in a primary diagnosis of hypogonadism was unchanged, the amount of monthly prescriptions for both injectable and transdermal testosterone formulations decreased significantly following the FDA safety advisory.
Back to 2017 Program
|
|
|
|






