Back to 2017 Program
Endothelialization of a 3D Tissue-Engineered Human Vaginal Mucosa for Implantation
Weronika Jakubowska, MD-MSc Student, Stéphane Chabaud, PhD., Ingrid Saba, PhD., Stéphane Bolduc, MD.
LOEX/ Université Laval, Quebec, QC, Canada.
INTRODUCTION
Tissue engineering of autologous vaginal mucosa opens the door to new surgical applications for vaginal reconstruction for paediatric patients with congenital urogenital abnormalities such as the Mayer-Rokitansky-Küster-Hauser syndrome (MRKH). Vascularization of tissue-engineered constructs represents a major challenge as graft survival and success rate highly depend on it. In this study, we aim at reconstructing a pseudo-capillary network within a tissue-engineered vaginal mucosa, free of exogenous materials, using the self-assembly technique. METHODS
Vaginal stromal and epithelial cells were isolated from healthy donors’ biopsies. Vaginal stromal cells were co-seeded with endothelial cells derived from a human umbilical cord vein (HUVEC). The self- assembly technique relies on the intrinsic properties of fibroblasts to secrete their own extracellular matrix in the presence of ascorbic acid. Different cell culture techniques were tested to determine the one that is most adapted for the vascularization of the vaginal mucosa while retaining good mechanical properties: classical self-assembly (SA), reseeding (RS) and a hybrid SA/RS method. At week two of culture, the conditions that required reseeding had a second seeding of vaginal fibroblasts and HUVEC onto the newly formed stromal sheets. The culture was continued for a total of four weeks for all conditions. After four weeks, vaginal epithelial cells were seeded on top of most stromal sheets and they remained in submerged conditions for one additional week to ensure epithelial cell proliferation. In the SA and SA/RS conditions, one epithelialized sheet was stacked with a non- epithelialized one to form constructs. Differentiation of the vaginal epithelium required an additional three weeks at the air-liquid interface for all conditions. Furthermore, mechanical uniaxial strength tests were performed on all conditions to evaluate their mechanical properties. RESULTS
Firstly, this reconstructed vaginal mucosa model closely mimics native tissue and undergoes adequate vaginal epithelium differentiation (Figure 1). Secondly, the biomechanical properties of the reconstructed tissues demonstrate mechanical resistance values that are suitable for implantation in an animal model. Thirdly, the presence of a pseudo-capillary network has been confirmed by immunofluorescence using endothelial cell specific markers such as PECAM-1/CD31 and Von Willebrand factor. Additionally, the vascular maturity of the capillary-like network we assessed by the presence of NG2 positive cells, a pericyte marker, in the constructs produced by the RS and SA/RS conditions. CONCLUSION
Our results have demonstrated that the use of a combined SA/RS technique is most adapted for the pre-vascularization of the vaginal mucosa as it generates more mature capillary networks within the constructs and has intermediate mechanical properties that are interesting for surgical handling.
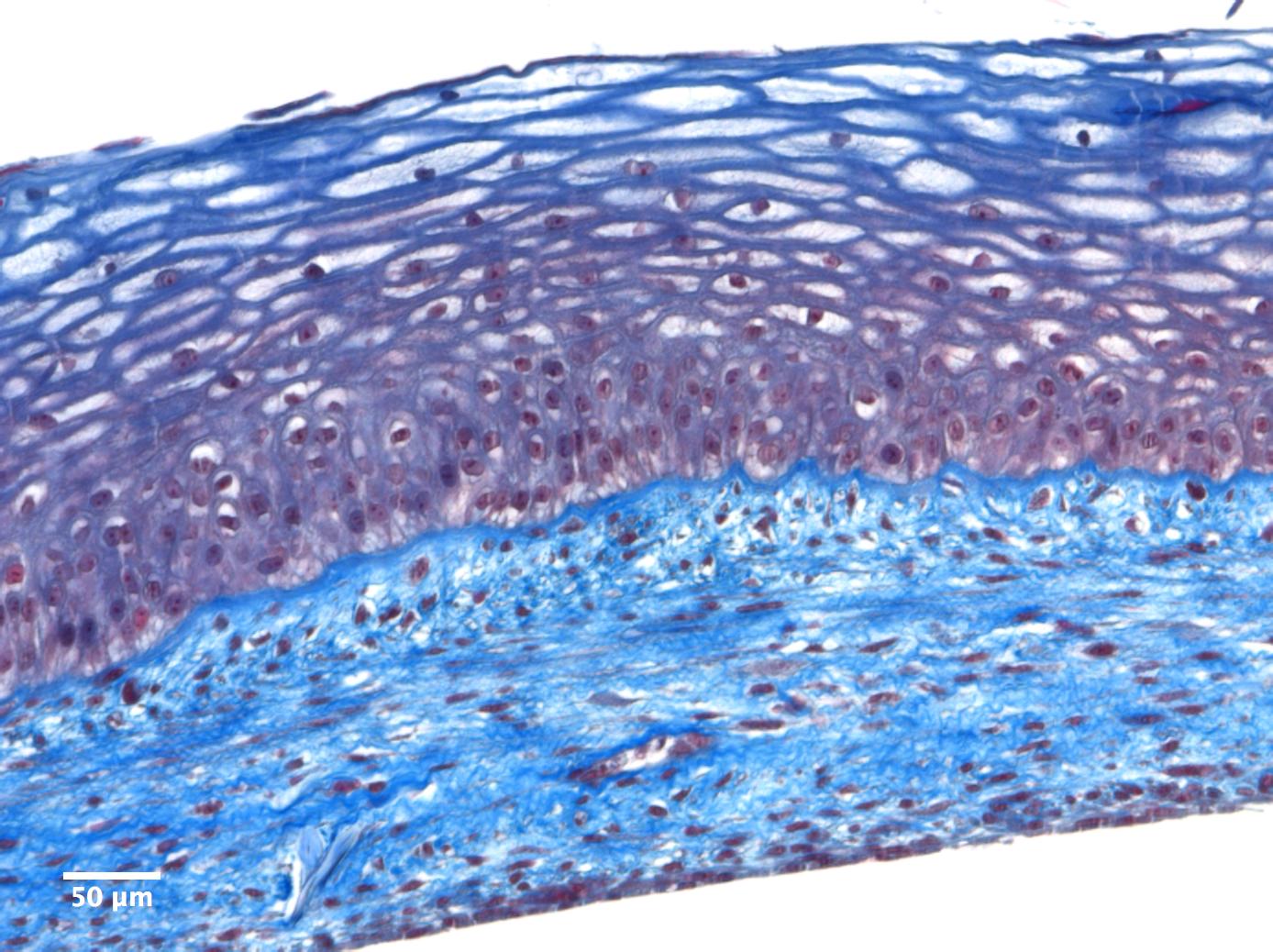
Figure1: Masson’s Trichrome stain of an endothelialized reconstructed model of the vaginal mucosa produced with the self-assembly technique.
Back to 2017 Program
|
|
|
|




