Back to 2025 Abstracts
IPP Infection Rates with 0.05% Chlorhexidine Gluconate (Irrisept®) Differ by Device Manufacturer: Initial Results from a Large Multi-Institutional Cohort
S Ivan, MD
1, Muhammed Hammad, MD
2, Elia Chawareb, md
3, Faysal Yafi, MD
3, Thairo Pereira, MD
4, Helen Bernie, MD
4, Stephen Harrington, MD
5, Richard Bellemare, MD
5,
Ambrose Orr, MD5, Nicholas Moll, MD
5, Aaron Lentz, MD
6, Alfredo Suarez-Sarmiento, MD
7, Paul Perito, MD
7, Tung-Chin Hsieh, MD
8, Matthew Ziegelmann, MD
9, Martin Gross, MD
5, Jay Simhan, MD
1.
1Fox Chase Cancer Center, Philadelphia, PA, USA,
2University of California, Irvine, Irvine, IN, USA,
3University of California, Irvine, Irvine, CA, USA,
4Indiana University, Indianapolis, IN, USA,
5Dartmouth Hitchcock Medical Center, Lebanon, NH, USA,
6Duke University, Durham, NC, USA,
7Perito Urology, Coral Gables, FL, USA,
8University of California, San Diego, San Diego, CA, USA,
9Mayo Clinic, Rochester, MN, USA.
BACKGROUND:Dilute 0.05% Chlorhexidine Gluconate (CHG) has gained popularity as an irrigant for inflatable penile prosthesis (IPP) as well as an dip for hydrophilic coated implants. Results from in vitro studies conflict on the efficacy of CHG for these purposes. We aimed to evaluate the efficacy of CHG as a dip and irrigant in clinical practice in a large multicenter collaboration.
METHODS:We conducted a retrospective analysis of 1,604 patients undergoing IPP placement across 12 referral centers. This was comprised of patients who underwent IPP placement with CHG as an irrigant or dip and an equal number of consecutive control patients where CHG was not used. Data collection spanned from January 1, 2017 to March 1, 2024. The primary outcome was explant for postoperative infection. Incidence of infection was compared across patient populations using chi-squared testing.
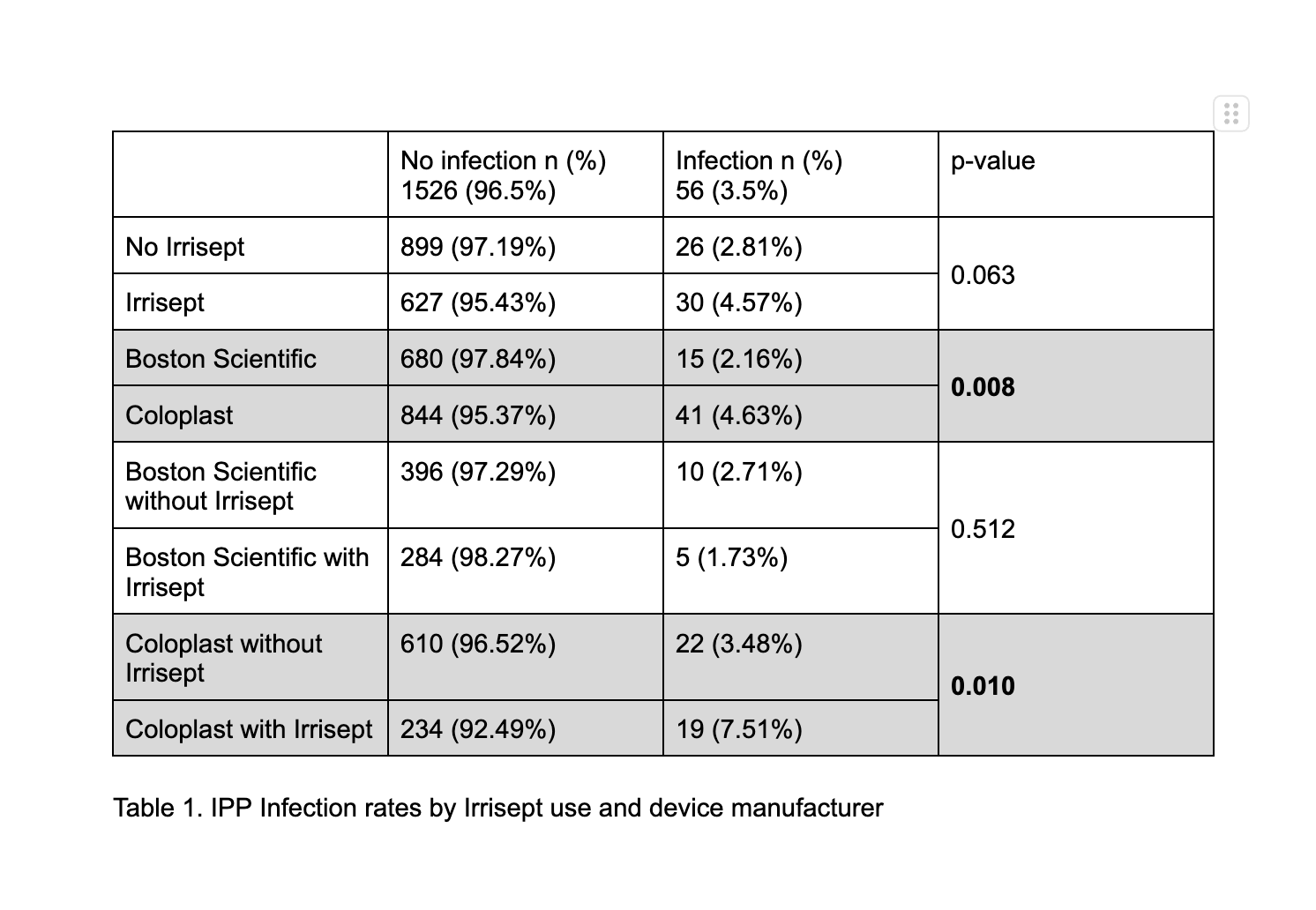
RESULTS: 927 (57.8%) cases used CHG as an irrigant or dip and 677 (42.2%) did not. CHG and non-CHG populations were similar in age, diabetes, coronary artery disease, smoking status, and BMI. In the entire cohort, the infection rate was 56 cases (3.5 %). CHG cases had an infection rate of 4.57% compared to 2.81% in non-CHG cases (p=0.063). Subgroup analysis by device manufacturer, the infection rate was higher in the Coloplast group compared to Boston Scientific (4.63% v. 2.16%, p=0.008). This corresponded to an elevated incidence of infection in Coloplast cases with CHG use, 7.51% compared to 3.48% in non-CHG Coloplast cases (p=0.010) There was no difference in infection rate for Boston Scientific cases with CHG use. (Table 1)
CONCLUSIONS:Initial results from a large multi institutional collaborative demonstrate elevated IPP infection rates when CHG is used with hydrophilic Coloplast devices.
Back to 2025 Abstracts
