Back to 2025 Abstracts
Nanoknife electroporation for the ablation of prostate tissue in intermediate-risk prostate cancer patients: Functional Outcomes from the PRESERVE trial
Jonathan S. Fainberg, MD MPH.
Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Background: Irreversible electroporation (IRE) is a tissue ablation method using high-voltage electrical pulses to change cell membrane permeability, leading to cell death. PRESERVE (NCT04972097) evaluated the safety and effectiveness of the NanoKnife System to ablate prostate tissue in intermediate-risk prostate cancer (PCa). We report the study’s functional outcomes.
Methods: PRESERVE is a prospective, non-randomized trial which included 121 patients across 17 US centers meeting key inclusion criteria: age>50 with organ-confined PCa, clinical stage ≤T2c, prostate-specific antigen (PSA) ≤15 ng/mL or PSA density<0.15 ng/mL
2 if PSA>15 ng/mL, and Gleason score 3+4 or 4+3. Table 1 lists patient and procedure characteristics. Patient-reported outcomes were collected through 12 months post-IRE.
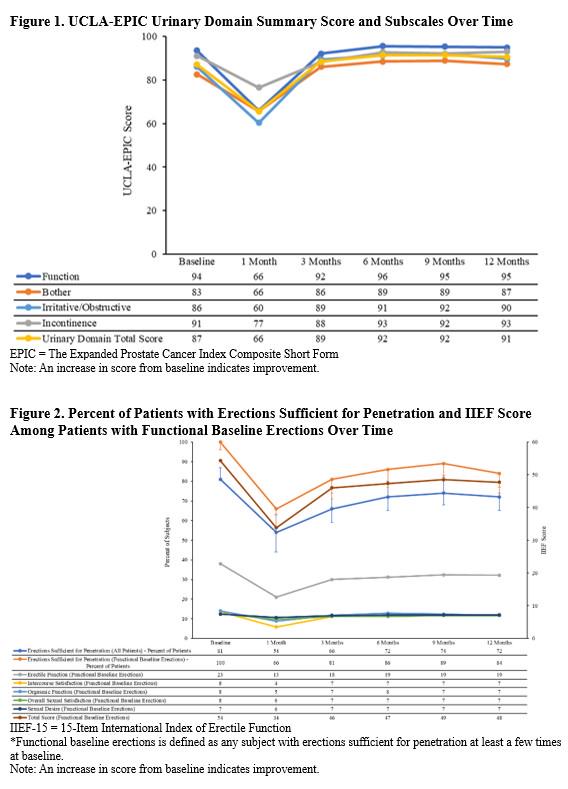
Results: The urinary domain score of the UCLA-EPIC questionnaire showed a mean of 87 at baseline and improved 12-month score of 91 (Figure 1). UCLA-EPIC function, bother, irritative/obstructive, and incontinence subscales also showed mean score improvements from baseline to 12 months post-procedure (Figure 1). Among patients (81%) with functional baseline erections, 72% retained erections sufficient for penetration at 12 months (Figure 2). All subscales of the IIEF-15 questionnaire approached baseline scores at 12 months (Figure 2).
Conclusions: PRESERVE demonstrates beneficial functional outcomes using the NanoKnife System for the ablation of prostate tissue in patients with intermediate-risk PCa. This device may provide an alternative to radical treatments while preserving functional outcomes.
| | | | | | | | | | | | | |
| Table 1. Patient and Procedure Characteristics | | |
| Characteristic | | | | | | Study PopulationN=121 | | |
| | | |
| Demographics | | | | | | | | |
| Race [n (%)] | | | | Asian | | 7 (5.8) | | |
| | | | | Black or African American | | 10 (8.3) | | |
| | | | | White | | 100 (82.6) | | |
| | | | | Other | | 1 (0.8) | | |
| | | | | Unknown | | 3 (2.5) | | |
| | | | | | | | | |
| Ethnicity [n (%)] | | | | Hispanic or Latino | | 4 (3.3) | | |
| | | | | Not Hispanic or Latino | | 110 (91) | | |
| | | | | Not Reported | | 3 (2.5) | | |
| | | | | Unknown | | 4 (3.3) | | |
| | | | | | | | | |
| Age at Informed Consent (years) | | | | Mean (std)MedianQ1, Q3 | | 67 (7)6761, 72 | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Disease Characteristics | | | | | | | | |
| AJCC Primary Tumor Stage at Screening [n (%)] | | | | T1b | | 1 (0.83) | | |
| | | | | T1c | | 103 (85) | | |
| | | | | T2a | | 13 (11) | | |
| | | | | T2b | | 4 (3.3) | | |
| | | | | | | | | |
| Grade Group at Screening [n (%)] | | | | 2 | | 97 (80) | | |
| | | | | 3 | | 24 (20) | | |
| | | | | | | | | |
| Lesion Regional Location [n (%)] | | | | n | | 120 | | |
| | | | | Apex | | 49 (41) | | |
| | | | | Base | | 18 (15) | | |
| | | | | Midline | | 53 (44) | | |
| | | | | | | | | |
| Lesion Anterior/Posterior Location [n (%)] | | | | n | | 120 | | |
| | | | | Posterior | | 70 (58) | | |
| | | | | Anterior | | 50 (42) | | |
| Procedure Characteristics | | | | | | | | |
| Duration of Procedure (Minutes) | | | | nMean (std)MedianQ1, Q3Min, Max | | 11654.1 (23.15)50.038.0, 63.518.0, 128.0 | | |
| | | | | | | | | |
| Number of Probes Used | | | | nMean (std)MedianQ1, Q3Min, Max | | 1214.3 (0.69)4.04.0, 5.03.0, 6.0 | | |
| | | | | | | | | |
| Lesion Location Directionality Section 1 | | | | n | | 120 | | |
| | | | | Apex | | 49 (40.8) | | |
| | | | | Base | | 18 (15.0) | | |
| | | | | Midline | | 53 (44.2) | | |
| | | | | | | | | |
| Lesion Location Directionality Section 2 | | | | n | | 120 | | |
| | | | | Anterior | | 50 (41.7) | | |
| | | | | Posterior | | 70 (58.3) | | |
| | | | | | | | | |
| Ultrasound/MRI Fusion Device During Procedure | | | | n | | 121 | | |
| | | | | Yes | | 105 (86.8) | | |
| | | | | No | | 16 (13.2) | | |
AJCC = American Joint Committee on Cancer; PSA = prostate-specific antigen
Back to 2025 Abstracts
