Back to 2025 Abstracts
The Phase 3 Open-Label COURAGE Extension Study to Evaluate the Long-Term Safety and Efficacy of Vibegron in Men With Overactive Bladder Symptoms on Pharmacotherapy for Benign Prostatic Hyperplasia
David R. Staskin, MD1, Janet Owens-Grillo, PhD, MS
2, Elizabeth Thomas, MS
2, Kenneth M. Peters, MD
3, Eric S. Rovner, MD
4, Salim Mujais, MD
2.
1Tufts University School of Medicine, Boston, MA, USA,
2Sumitomo Pharma America, Inc., Marlborough, MA, USA,
3Oakland University William Beaumont School of Medicine, Rochester, MI, Corewell Health William Beaumont University Hospital, Royal Oak, MI, USA,
4Medical University of South Carolina, Charleston, SC, USA.
Introduction: In the phase 3 COURAGE trial, vibegron, a β
3-adrenergic receptor agonist, was associated with significant improvements vs placebo in all primary and secondary efficacy endpoints and was safe and well tolerated in men with overactive bladder (OAB) symptoms on pharmacotherapy for benign prostatic hyperplasia (BPH). Long-term safety and efficacy of vibegron were evaluated in an open-label extension (OLE) study.
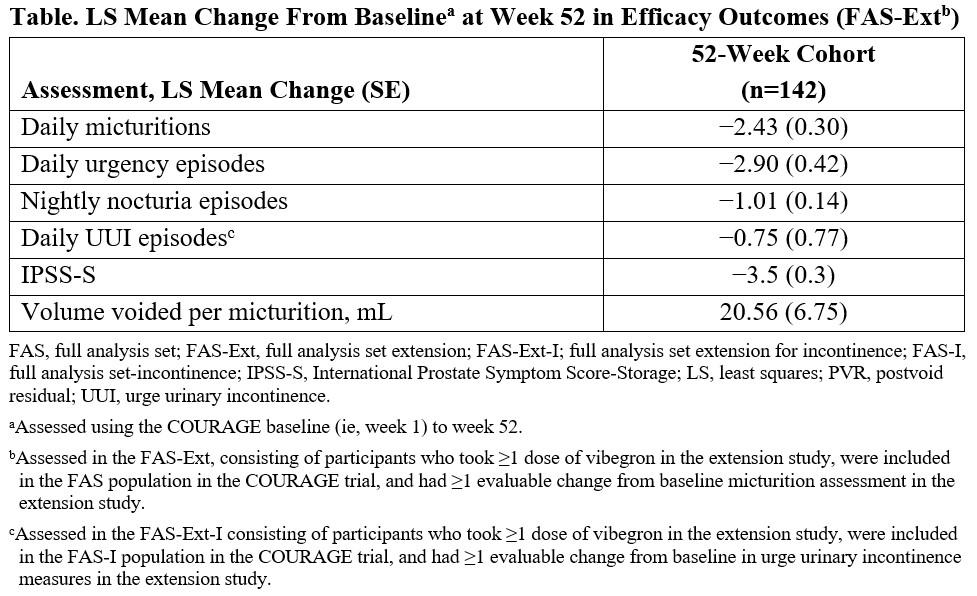
Materials & Methods: Participants who completed the COURAGE trial (once-daily vibegron 75 mg vs placebo for 24 weeks) received vibegron for 28 weeks in the extension. The primary outcome was safety (adverse events [AEs], clinical laboratory assessments, vital signs, postvoid residual urine volume [PVR], and International Prostate Symptom Score [IPSS] total score). Secondary outcomes were change from baseline to week 52 in mean daily micturitions, urgency episodes, nightly nocturia episodes, urge urinary incontinence episodes, IPSS-Storage, and volume voided/micturition.
Results: Overall, 276 participants who completed COURAGE enrolled (vibegron, n=142 [52-week cohort]; placebo, n=134); 90.6% completed the OLE. Nine participants (3.3%) discontinued vibegron owing to AEs. In the 52-week cohort, the incidences of AEs were similar during double-blind (35.9%) and open-label (29.6%) treatment. The most commonly reported AEs were hypertension (6.3%), COVID-19 (5.6%), and hepatic enzyme increased (2.1%). There were no clinically relevant changes in laboratory parameters or vital signs. No participant sustained PVR ≥200 mL at ≥2 consecutive assessments. In the 52-week cohort, median (IQR) IPSS total score decreased from baseline to week 52 (−7.0 [−11.0, −2.0]). Improvements in all secondary outcomes were observed for participants receiving vibegron for 52 weeks (
Table).
Conclusions: Vibegron demonstrated favorable long-term safety and efficacy for up to 52 weeks in men with OAB symptoms receiving pharmacologic treatment for BPH.
Back to 2025 Abstracts
