|
Back to 2014 Annual Meeting Abstracts
TRPV3 Channels in Mouse Urinary Bladder Function: A possible therapeutic target for overactive bladder
Michael K. Lam, MD1, Travis Mann-Gow, BS1, Katarina Zvarová, MD, PhD1, Benjamin King, MD1, Magdalene M. Moran, PhD2, Mark T. Nelson, PhD1, Peter Zvara, MD, PhD1.
1University of Vermont, Burlington, VT, USA, 2Hydra Biosciences, Cambridge, MA, USA.
BACKGROUND: TRP channels have been shown to act as mechanosensory and pain receptors in a wide variety of organ systems. Despite their presence in the bladder, to date their pharmacologic modulation has not been shown to be effective in the treatment of overactive bladder and lower urinary tract symptoms. This study utilizes wild-type (WT) and TRPV3-knock out (KO) mice, coupled with novel compounds to clarify TRPV3’s role in the regulation of bladder function.
METHODS: Immunohistochemistry was performed on bladder wall and dorsal root ganglia (DRG). Myograph studies measuring isometric muscle contractility were used to evaluate the response to TRPV3, TRPV1, TRPA1, and neurokinin (NK)-specific agonist and antagonists. Cystometry was recorded in awake mice during intravesical administration of 0.9% NaCl, 0.25% acetic acid (AA) in normal saline, and TRPV3 antagonist.
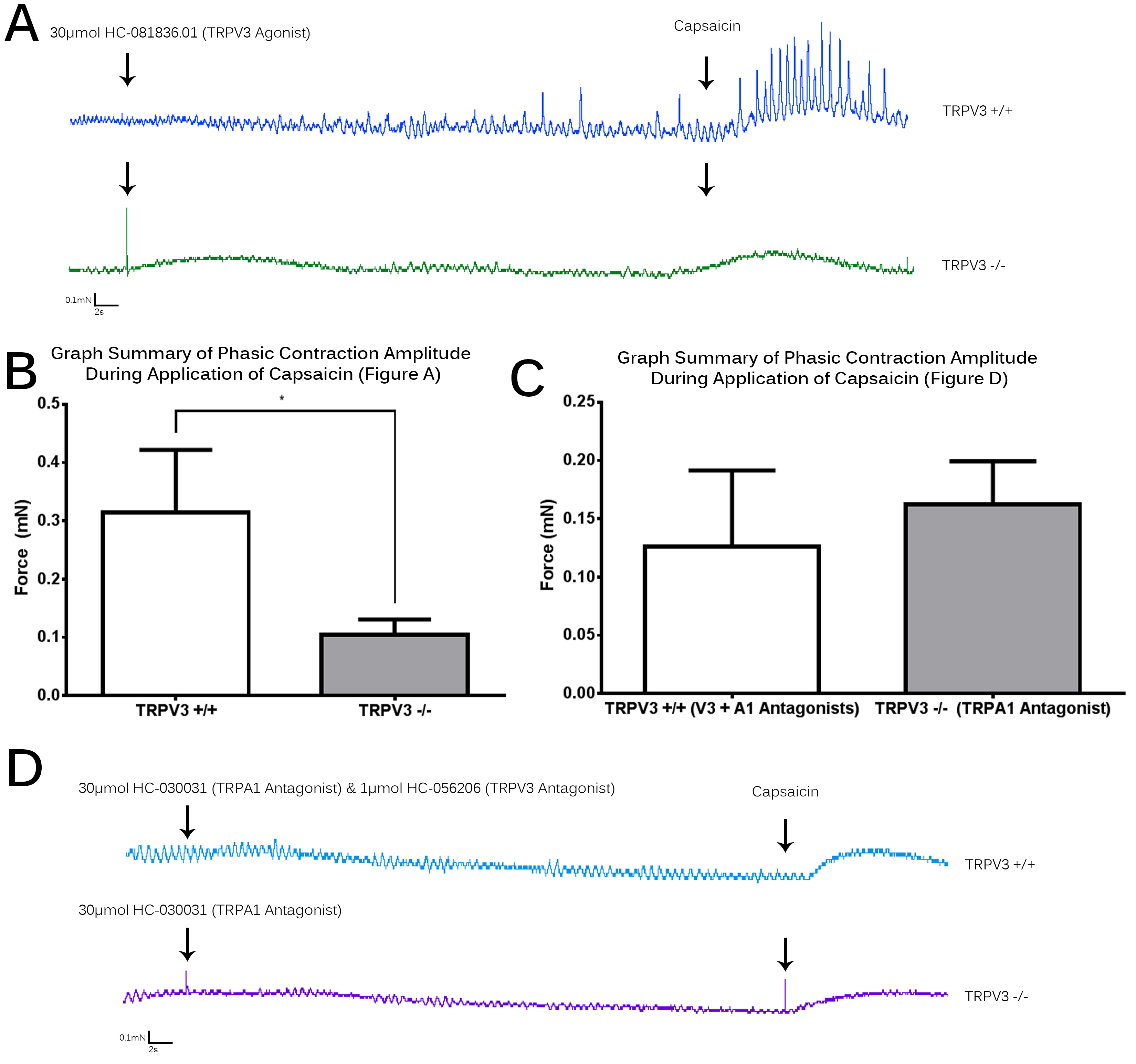
RESULTS: TRPV3 expression was documented in the urothelium, suburothelium, bladder smooth muscle, and L6 DRG neurons. TRPV3 expression increased in animals after partial bladder outlet obstruction. TRPV3 agonists increased the amplitude of baseline phasic contractions in bladder muscle wall strips (n=7, p < 0.01). This was potentiated with simultaneous TRPV3 and TRPV1 activation using capsaicin. TRPV3 suppression with TRPV3 antagonists had no effect on baseline contractility, however combination of TRPV3 and TRPA1 antagonists decreased detrusor muscle tone (n=5, p < 0.01). NK receptor antagonists suppressed phasic contractions during application of TRPV3 agonists (n=6, p < 0.05). Using awake cystometry, the increased intravesical pressure and voiding frequency seen with 0.25% AA infusion reversed towards normal with addition of TRPV3 antagonists (n=5, p < 0.01). Treatment with agonist and capsaicin to muscle strips from global TRPV3-KO mice demonstrated a significant change as compared to TRPV3-WT mice. (Figures A and B, n=4, p < 0.01). Adding TRPA1 antagonists to TRPV3-KO mice or TRPV3 and A1 antagonists to WT mice both suppressed the amplitude of phasic contractions (Figures C and D, n=4, p < 0.01).
CONCLUSIONS: These data suggest that TRPV3 plays a critical role in mediating bladder function through its effect on non-voiding phasic contractions. TRPV3 agonists stimulate phasic contractions, demonstrating a detrusor overactivity. TRPV3-specific compounds appear to act through the release of neurokinins, and we demonstrate evidence that different TRP channels act in concert. Understanding and targeting these pathways may provide possible future avenues for treatment of overactive bladder.
Back to 2014 Annual Meeting Abstracts
|

|

