|
|
 |
Back to Annual Meeting Program
Composite Responders Showed Improvement in Both Penile Curvature Deformity and Symptom Bother in Two Large Double-Blind, Randomized, Placebo-Controlled Phase 3 Studies of Collagenase Clostridium Histolyticum in the Treatment of Peyronie’s Disease
Robert Feldman, MD1, Wayne Hellstrom, MD2, Ted Smith, PhD3, Sue Hobson, RN3, James Tursi, MD3, Guy T. Bernstein, MD4.
1Connecticut Clinical Research Center and Urology Specialists, Middlebury, CT, USA, 2Tulane University Health Sciences Center, New Orleans, LA, USA, 3Auxilium Pharmaceuticals, Inc., Chesterbrook, PA, USA, 4Center for Urologic Care Urology Health Specialists, Bryn Mawr, PA, USA.
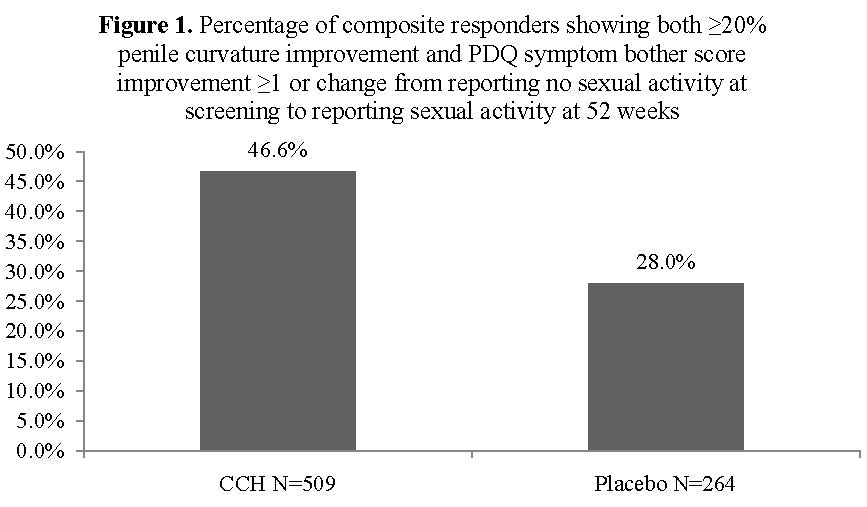
BACKGROUND: The Investigation for Maximal Peyronie’s Reduction Efficacy and Safety Studies (IMPRESS) I and II examined the clinical efficacy of collagenase clostridium histolyticum (CCH) in subjects with Peyronie’s disease (PD). These large, international, identical phase 3 randomized, double-blind, placebo-controlled studies examined CCH treatment outcomes in penile curvature deformity and PD symptom bother domain using the Peyronie’s disease questionnaire (PDQ) from baseline to 52 weeks. Composite responders showed both a ≥20% improvement in penile curvature deformity and an improvement of ≥1 in the symptom bother PDQ score, or a change from reporting no sexual activity at screening to reporting sexual activity. The percentage of subjects who were composite responders was compared between CCH treatment and placebo groups.
METHODS: The IMPRESS I (N=417) and II (N=415) phase 3 studies examined CCH treatment through a maximum of up to 4 treatment cycles, each separated by a 6-week period. Subjects received up to 8 injections into the Peyronie’s plaque of 0.58 mg CCH, two injections per cycle separated by approximately 24 to 72 hours, with the second injection in each cycle followed 24-72 hours later by plaque modeling. Subjects were stratified by degree of penile curvature deformity at baseline (30° to 60° vs 61° to 90°) and randomized to CCH or placebo (2:1 in favor of CCH). A post-hoc meta-analysis combined data from the two identical studies.
RESULTS: The group of men who received CCH treatment had a significantly greater percentage of composite responders compared with the placebo group at Week 52 (p<0.0001; Figure 1).
CONCLUSIONS: The IMPRESS I and II studies support the clinical efficacy of CCH treatment compared with placebo for both the physical and psychological aspects of PD. CCH treatment resulted in a greater percentage of men showing improvement in both penile curvature deformity and PD symptom bother.
Back to Annual Meeting Program
|