|
|
 |
Back to Annual Meeting Program
Metformin disrupts tumor metabolism in prostate cancer cell lines by rearranging glucose and glutamine utilization.
Gregory J. Wirth, MD1, Sarah-Maria Fendt, PhD2, Eric L. Bell, PhD2, Mark A. Keibler, PhD3, Gregory Stephanopoulos, PhD3, Aria F. Olumi, MD1.
1Massachusetts General Hospital, Boston, MA, USA, 2Massachusetts Institute of Technology, Cambridge, MA, USA, 3Massachusetts Institute of Technology, Cambridge, MA, USA.
Background: Excess nutrient metabolic states such as obesity and hyperinsulinemia are associated with prostate cancer progression. Metformin is the most widely prescribed oral antidiabetic agent and has been shown to reduce the risk and mortality of multiple cancer types. Current evidence suggests a direct antitumoral effect by inhibition of mitochondrial respiration, and an indirect effect by reduction of blood glucose and insulin levels. However, its effect of prostate cancer bioenergetics is poorly understood. We aim to investigate how Metformin rearranges metabolic fluxes of prostate cancer cells and how this rearrangement can be targeted for cancer treatment.
Methods: Experiments were performed in 3 prostate cancer cell lines (PC3, LNCaP, DU145) treated with 0.5-2.5mM metformin. Cell respiration was assessed by measuring oxygen consumption, glucose uptake and lactate secretion. 13C flux analysis allowed the assessment of glucose and glutamine utilization and metabolic pathway activity. Proliferation was quantified with cell counts and protein expression with Western blots.
Results: Metformin increased glucose uptake and lactate secretion while reducing oxygen consumption in a dose-dependent manner, suggesting a shift form respiratory energy production to fermentation. Accordingly, cell lines that strongly relied on respiration were most sensitive to metformin. Flux analysis revealed that in untreated cells, glucose was directed towards the TCA cycle and constituted the major carbon source for fatty acid synthesis. Metformin inhibited glucose fueling of the TCA cycle. Instead, glutamine was funneled into the TCA cycle and used for fatty acid synthesis. Flux analysis showed that this switch was achieved by a partial reversal of the TCA cycle from the usual oxidative metabolism to a reductive flux. As the enzyme glutaminase is necessary to divert glutamine into the mitochondria and the TCA cycle, we combined Metformin with an allosteric inhibitor of glutaminase and found a synergistic, anti-proliferative effect in all cell lines. Conclusion: Metformin induces a profound rearrangement of central metabolism in prostate cancer cell lines. Cells shift ATP synthesis from respiration to less efficient fermentation and resort to a reductive TCA cycle to uphold biosynthetic processes. Inhibition of this secondary bioenergetic pathway with a glutaminase inhibitor achieves a synergistic anti-tumor effect with metformin. These alterations of metabolic fluxes may ultimately represent new targets for cancer therapy. 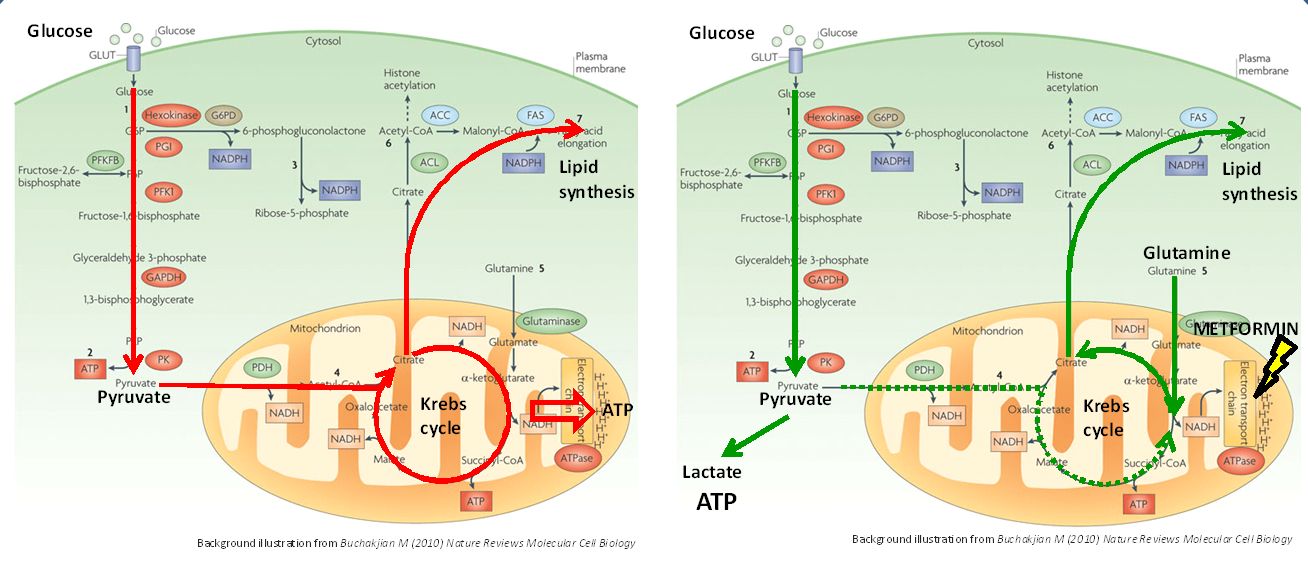
Back to Annual Meeting Program
|