|
|
 |
Back to Annual Meeting Program
Overall Survival Benefit with Sipuleucel-T by Baseline PSA: Exploratory Analysis from the IMPACT Trial
Philip W. Kantoff, MD1, Gerald Chodak, MD2, Paul Schellhammer, MD3, James B. Whitmore, PhD4, Robert B. Sims, MD4.
1Dana-Farber Cancer Institute of Harvard Medical School, Boston, MA, USA, 2Louis A Weiss Memorial Hospital, Chicago, IL, USA, 3Eastern Virginia Medical School / Urology of Virginia, Norfolk, VA, USA, 4Dendreon, Seattle, WA, USA.
BACKGROUND: : In the IMPACT trial, sipuleucel−T reduced risk of death by 22.5% (P=0.032). A pre−specified subgroup analysis for baseline prognostic variables showed homogeneous treatment effects consistently favoring sipuleucel−T. In patients with baseline PSA below vs above the median, there was a trend toward greater treatment effect (HR=0.685 vs. 0.865). In this exploratory analysis, we further sub−divided baseline PSA into quartiles to evaluate potential treatment effect patterns.
METHODS: All randomized IMPACT patients (n=512) were categorized by baseline PSA quartile, ECOG, and other prognostic variables (LDH, PAP, ALP in bone−only disease, Hgb). Median overall survival (OS) and hazard ratio (HR) were estimated using Kaplan−Meier and Cox models, respectively.
RESULTS: Increasing baseline PSA quartile was associated with markers of advanced disease. Although there was inadequate power to demonstrate inter−quartile significance, data suggest a consistent treatment effect in all subsets and a trend toward an increased effect with lower baseline PSA. A similar benefit was suggested for other prognostic variables, with the exception of baseline Hgb.
CONCLUSIONS: This analysis supports a consistent OS benefit with sipuleucel−T across PSA quartiles, and suggests that patients with less advanced disease may benefit more from treatment with sipuleucel−T. 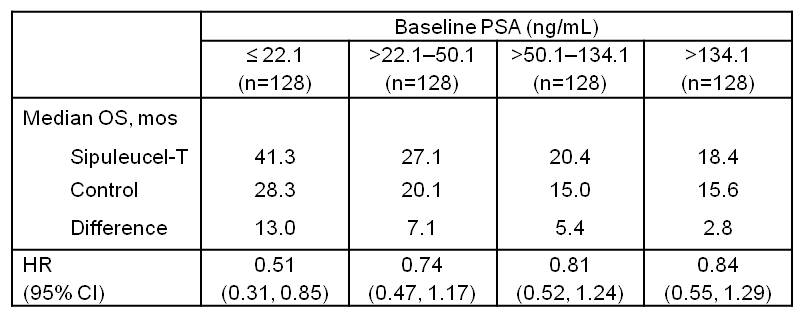
Back to Annual Meeting Program
|