|
Back to 2011 Program
A Once-daily Titratable Gel Formulation for Transdermal Oxybutynin Delivery for OAB
David R Staskin, MD3, Evan Goldfisher2, Kaushik Dave3
Tufts University School Of Medicine, Boston, MA; 2Hudson Valley Urology, Poughkeepsie, NY 3; Antares Pharma, Ewing, NJ
Introduction:
A prospective randomized double-blind placebo-controlled trial of a once-daily titrable dose transdermal oxybutynin gel (TTOG) formulation. To date there are no titrable transdermal agents for OAB.
Materials & Methods:
12 week study ages 19-89 years with symptoms of urgency (UUI) and/or mixed UI for >3 months. Inclusion: >1-2 urge episodes and >8 voids/day. Three treatment arms: 84mg and 56mg TTOG and placebo. Primary: change from baseline in (UI) using a 3-day diary. Secondary: change from baseline in urinary frequency and volume voided. Primary analysis: modified intent to treat. Diaries: baseline, and weeks 1,2,4,8, and 12. Statistics: transformation for group comparison (predefined). IRB approved. Gel formulation supplied (Anturol) and study funding by Antares Pharma, Inc., Ewing, NJ.
Results:
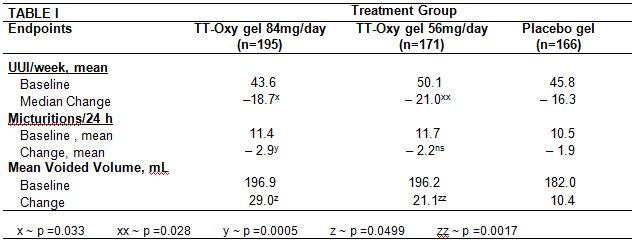
626 patients (87% female) were included: TTOG 84mg (N=214), 56mg (N=210), and placebo (N= 202). Both doses of TTOG were statistically superior to placebo for UUI reduction and volume voided; 84mg dose for urinary frequency (Table 1). AEs:mild to moderate / non-prompted rates of dry mouth n= 26 (12.1%)/84mg and n= 23 (11.0%)/56mg TTOG and n=10 (5.0%)/placebo. CNS AEs were similar between both active arms and placebo group.
Conclusions: This is the first report of a TTOG. Significant improvement noted for OAB symptoms at both doses. Side effects were mild to moderate with low levels of skin reactivity. TTOG provides an additional alternative for managing OAB symptoms.
Back to 2011 Program
|